Question: 1) (20 pt) Repeat the material balance distillation exercise from class (Example of 6-1 of Wankat) for n- pentane. Set up the full matrix based

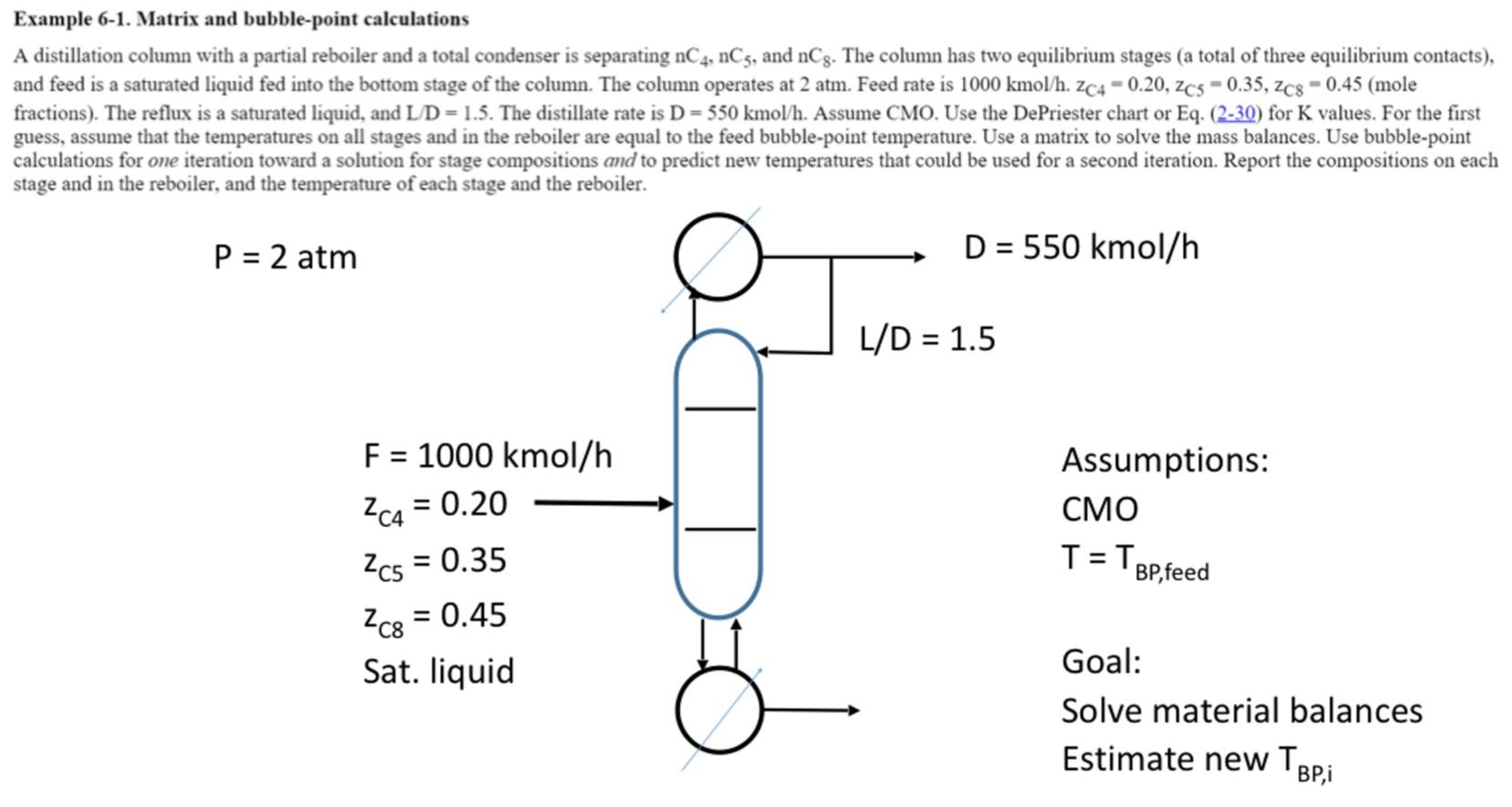
1) (20 pt) Repeat the material balance distillation exercise from class (Example of 6-1 of Wankat) for n- pentane. Set up the full matrix based on the initial assumed flowrates and K values. Then solve the matrix to get liquid flowrates using the Thomas algorithm (by hand or by a Matlab program (preferred)). a Example 6-1. Matrix and bubble-point calculations A distillation column with a partial reboiler and a total condenser is separating nC4, nC5, and nCg. The column has two equilibrium stages (a total of three equilibrium contacts), and feed is a saturated liquid fed into the bottom stage of the column. The column operates at 2 atm. Feed rate is 1000 kmol/h. Zc4 -0.20, Zcs -0.35, Zcs - 0.45 (mole fractions). The reflux is a saturated liquid, and LID=1.5. The distillate rate is D = 550 kmol/h. Assume CMO. Use the De Priester chart or Eq. (2-30) for K values. For the first guess, assume that the temperatures on all stages and in the reboiler are equal to the feed bubble-point temperature. Use a matrix to solve the mass balances. Use bubble-point calculations for one iteration toward a solution for stage compositions and to predict new temperatures that could be used for a second iteration. Report the compositions on each stage and in the reboiler, and the temperature of each stage and the reboiler. P = 2 atm D = 550 kmol/h L/D = 1.5 F = 1000 kmol/h = Assumptions: CMO - 2c4 = 0.20 2c5 = 0.35 Zc8 = 0.45 T=T Bp,feed Sat.liquid Goal: Solve material balances Estimate new TBP,i 1) (20 pt) Repeat the material balance distillation exercise from class (Example of 6-1 of Wankat) for n- pentane. Set up the full matrix based on the initial assumed flowrates and K values. Then solve the matrix to get liquid flowrates using the Thomas algorithm (by hand or by a Matlab program (preferred)). a Example 6-1. Matrix and bubble-point calculations A distillation column with a partial reboiler and a total condenser is separating nC4, nC5, and nCg. The column has two equilibrium stages (a total of three equilibrium contacts), and feed is a saturated liquid fed into the bottom stage of the column. The column operates at 2 atm. Feed rate is 1000 kmol/h. Zc4 -0.20, Zcs -0.35, Zcs - 0.45 (mole fractions). The reflux is a saturated liquid, and LID=1.5. The distillate rate is D = 550 kmol/h. Assume CMO. Use the De Priester chart or Eq. (2-30) for K values. For the first guess, assume that the temperatures on all stages and in the reboiler are equal to the feed bubble-point temperature. Use a matrix to solve the mass balances. Use bubble-point calculations for one iteration toward a solution for stage compositions and to predict new temperatures that could be used for a second iteration. Report the compositions on each stage and in the reboiler, and the temperature of each stage and the reboiler. P = 2 atm D = 550 kmol/h L/D = 1.5 F = 1000 kmol/h = Assumptions: CMO - 2c4 = 0.20 2c5 = 0.35 Zc8 = 0.45 T=T Bp,feed Sat.liquid Goal: Solve material balances Estimate new TBP,i
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


