Question: 1. (3 pts) Compound 1 contains the elements C,H, and O and gives the following combustion mass spec, and IR data: mass spec: M+m/z=136 IR:
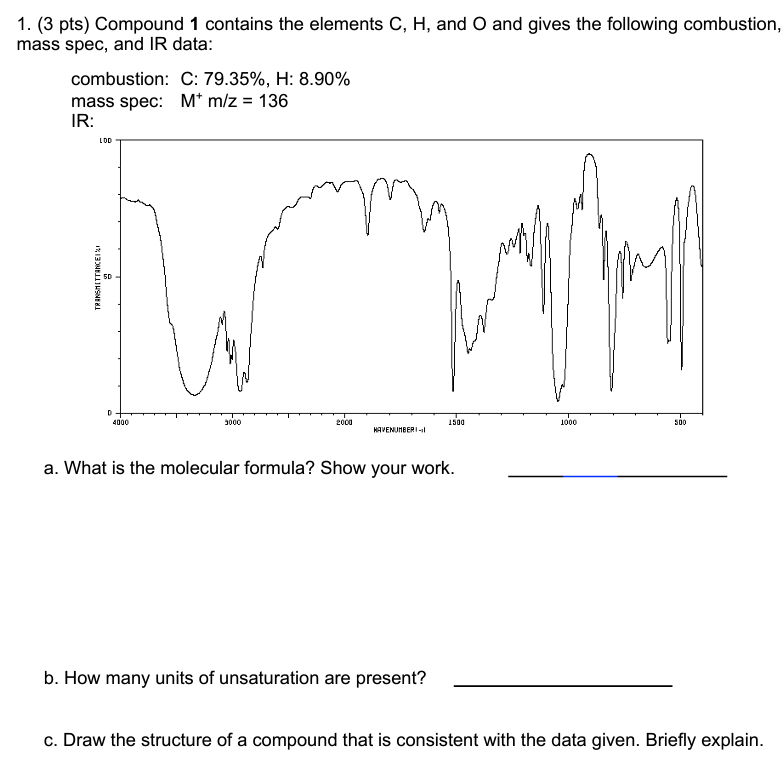
1. (3 pts) Compound 1 contains the elements C,H, and O and gives the following combustion mass spec, and IR data: mass spec: M+m/z=136 IR: a. What is the molecular formula? Show your work. b. How many units of unsaturation are present? c. Draw the structure of a compound that is consistent with the data given. Briefly explain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


