Question: 1. (30 points) The native (folded) and denatured (unfolded) forms of many proteins can be described by the simple two-state equilibrium: protein (native) + protein
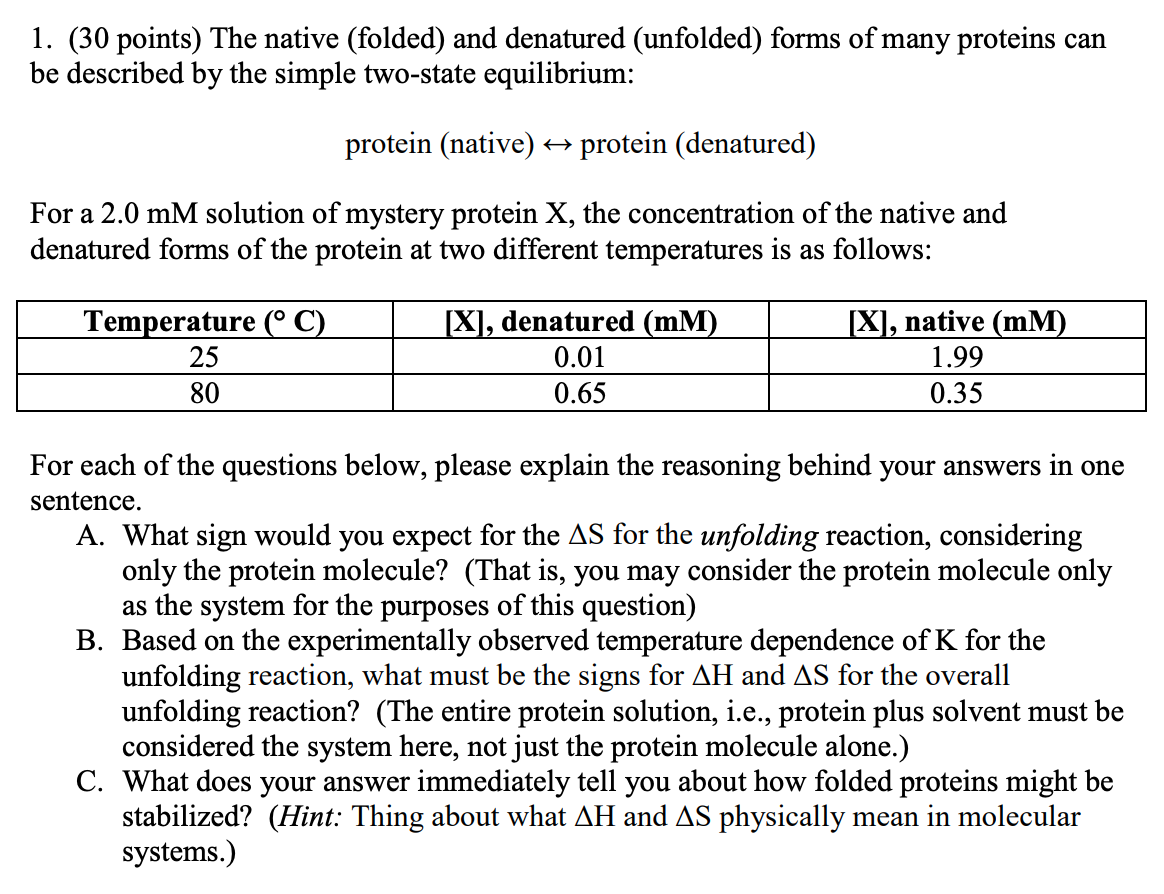
1. (30 points) The native (folded) and denatured (unfolded) forms of many proteins can be described by the simple two-state equilibrium: protein (native) + protein (denatured) For a 2.0 mM solution of mystery protein X, the concentration of the native and denatured forms of the protein at two different temperatures is as follows: Temperature (C) 25 80 [X], denatured (mm) 0.01 0.65 [X], native (MM) 1.99 0.35 For each of the questions below, please explain the reasoning behind your answers in one sentence. A. What sign would you expect for the AS for the unfolding reaction, considering only the protein molecule? (That is, you may consider the protein molecule only as the system for the purposes of this question) B. Based on the experimentally observed temperature dependence of K for the unfolding reaction, what must be the signs for AH and AS for the overall unfolding reaction? (The entire protein solution, i.e., protein plus solvent must be considered the system here, not just the protein molecule alone.) C. What does your answer immediately tell you about how folded proteins might be stabilized? (Hint: Thing about what AH and AS physically mean in molecular systems.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


