Question: = - 1 3/2 e The radial probability density of a hydrogen wavefunction in the 1s state is given by P(n) = 47tr2 (R18 (r))
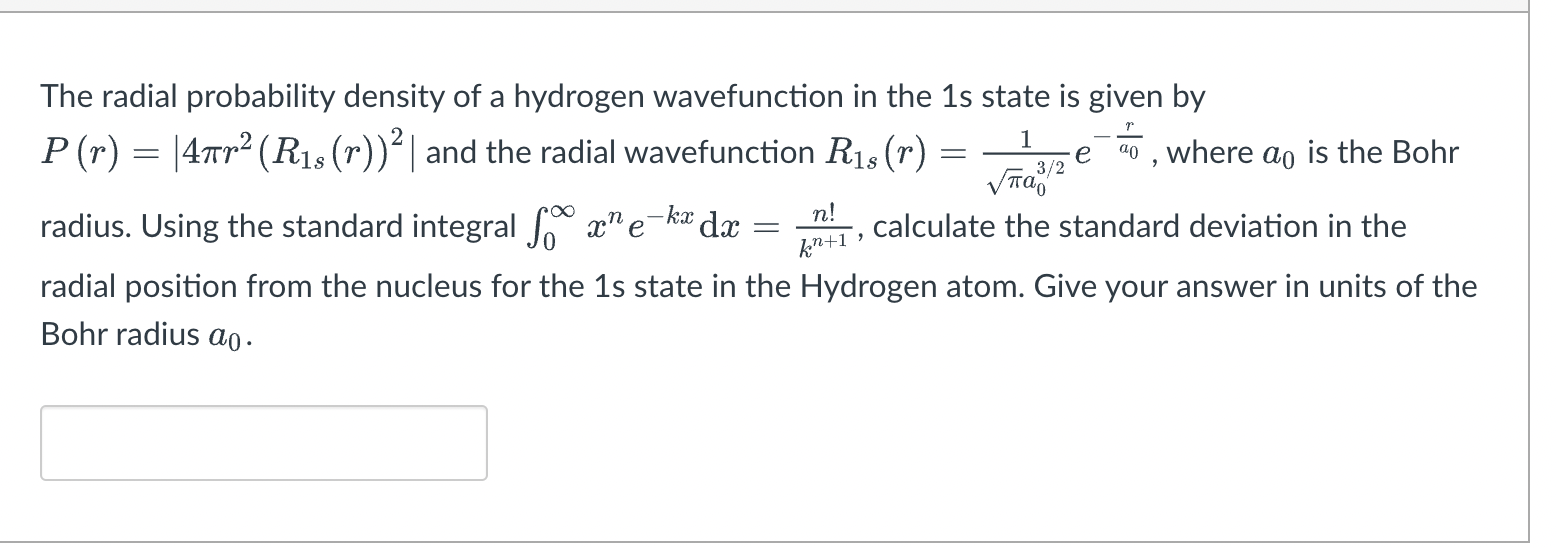
= - 1 3/2 e The radial probability density of a hydrogen wavefunction in the 1s state is given by P(n) = 47tr2 (R18 (r)) ? | and the radial wavefunction R18(r) = 20 , where ao is the Bohr radius. Using the standard integral So x" e ka dx calculate the standard deviation in the radial position from the nucleus for the 1s state in the Hydrogen atom. Give your answer in units of the Bohr radius ao. n! e = kn+1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


