Question: 1. (4 points) Please explain the following solubility trend using concepts appropriate for a 3000 level course. I am aware that we haven't explicitly covered
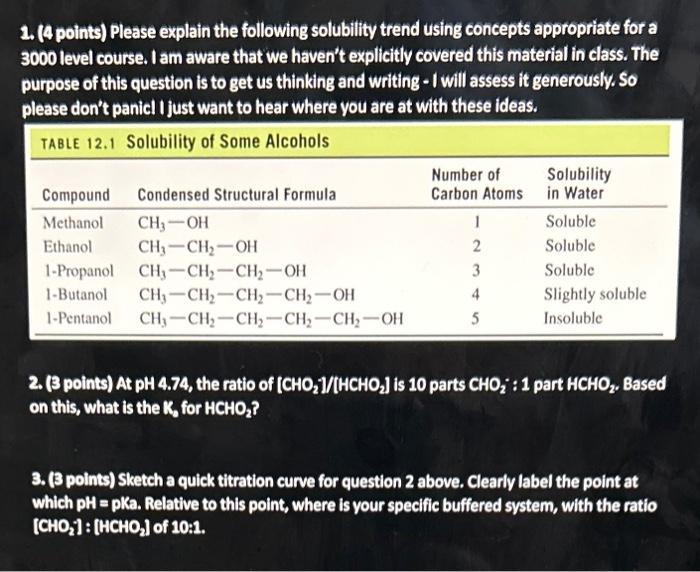
1. (4 points) Please explain the following solubility trend using concepts appropriate for a 3000 level course. I am aware that we haven't explicitly covered this material in class. The purpose of this question is to get us thinking and writing - I will assess it generously. So please don't panicl I just want to hear where you are at with these ideas. 2. (3 points) At pH4.74, the ratio of [CHO2]/(HCHO2] is 10 parts CHO2:1 part HCHO2, Based on this, what is the K2, for HCHO2 ? 3. (3 points) Sketch a quick titration curve for question 2 above. Clearly label the point at which pH=pKa. Relative to this point, where is your specific buffered system, with the ratio [CHO2]:[HCHO2] of 10:1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


