Question: 1. (40 pt) Create a complete stoichiometric table if the gas phase reaction takes place isothermally as: H2 + Nz+ - NHS 2 2 +
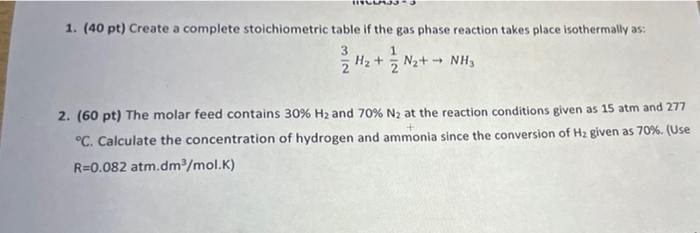
1. (40 pt) Create a complete stoichiometric table if the gas phase reaction takes place isothermally as: H2 + Nz+ - NHS 2 2 + 3 1 - 2. (60 pt) The molar feed contains 30% H2 and 70% N2 at the reaction conditions given as 15 atm and 277 C. Calculate the concentration of hydrogen and ammonia since the conversion of Hz given as 70%. (Use R=0.082 atm.dm/mol.K)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


