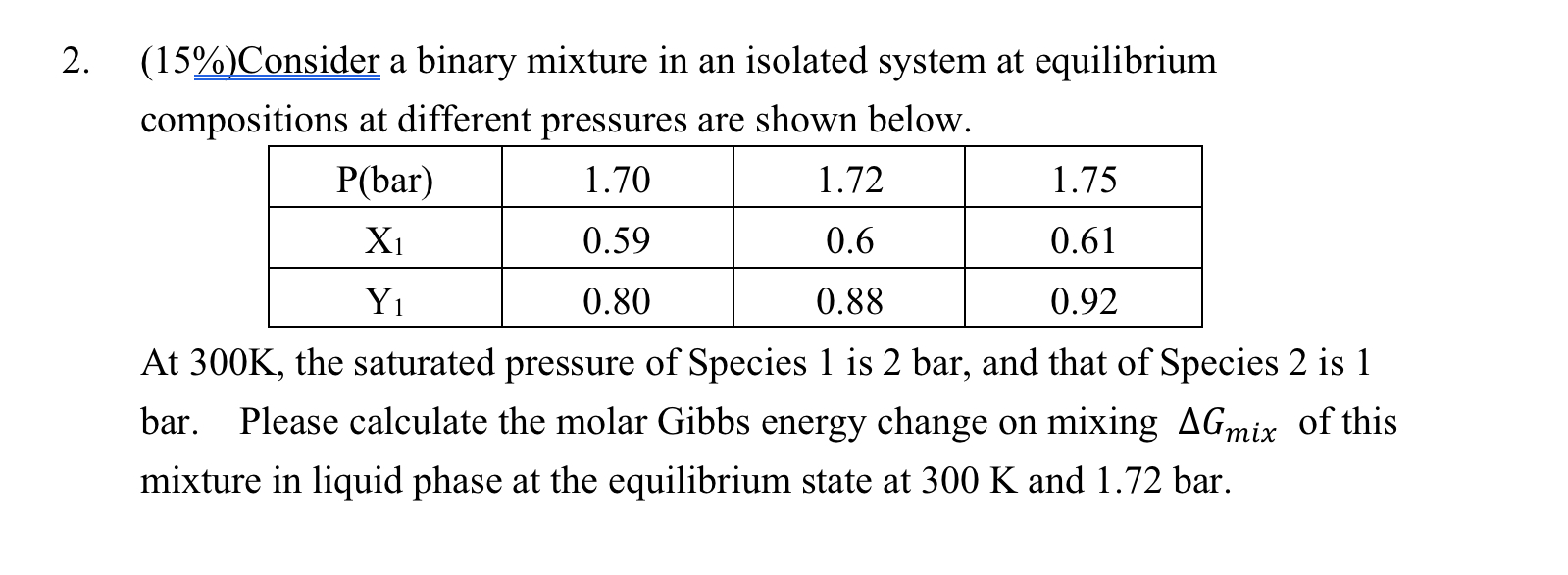
Question: ( 1 5 % ) Consider a binary mixture in an isolated system at equilibrium compositions at different pressures are shown bLow. At 3 0
Consider a binary mixture in an isolated system at equilibrium compositions at different pressures are shown bLow. At K the saturated pressure of Species is bar, and that of Species is bar. Please calculate the molar Gibbs energy change on mixing AGmix of this mixture in liquid phase at the equilibrium state at K and bar.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


