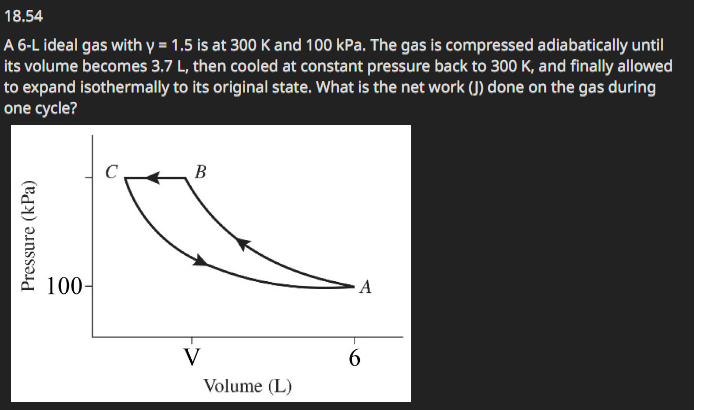
Question: 1 8 . 5 4 A 6 - L ideal gas with ( gamma = 1 . 5 ) is at 3
A L ideal gas with gamma is at K and kPa The gas is compressed adiabatically until its volume becomes L then cooled at constant pressure back to K and finally allowed to expand isothermally to its original state. What is the net work J done on the gas during one cycle?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


