Question: ( 1 9 ) * A distiller working in a Montreal Microdistillery is trying to get an improved understanding of his distillation process. He obtained
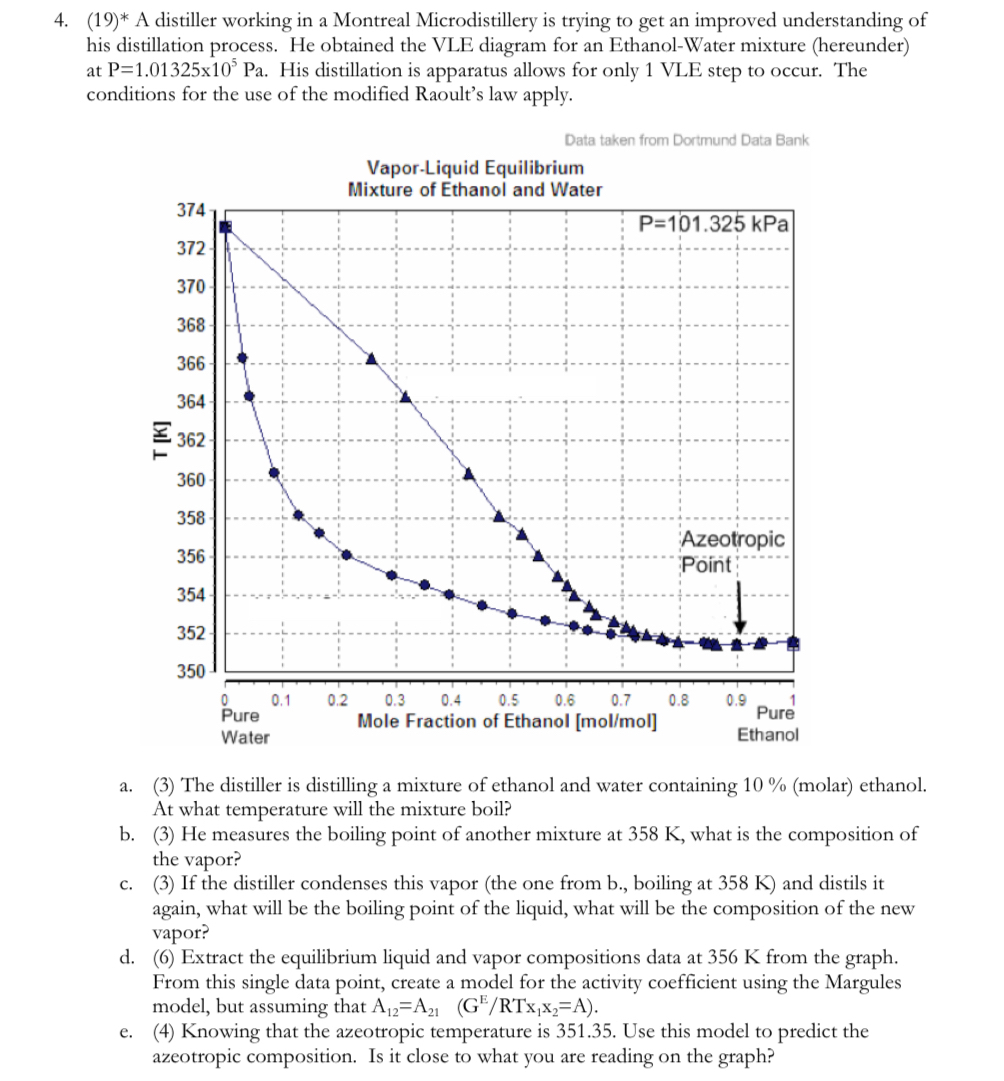
A distiller working in a Montreal Microdistillery is trying to get an improved understanding of his distillation process. He obtained the VLE diagram for an EthanolWater mixture hereunder at His distillation is apparatus allows for only VLE step to occur. The conditions for the use of the modified Raoult's law apply.
Data taken from Dortmund Data Bank
Vanor.l iauid Fauilihrium
a The distiller is distilling a mixture of ethanol and water containing molar ethanol. At what temperature will the mixture boil?
b He measures the boiling point of another mixture at what is the composition of the vapor?
c If the distiller condenses this vapor the one from b boiling at and distils it again, what will be the boiling point of the liquid, what will be the composition of the new vapor?
d Extract the equilibrium liquid and vapor compositions data at from the graph. From this single data point, create a model for the activity coefficient using the Margules model, but assuming that
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


