Question: # 1 . ( a ) Calculate the molar volume of chlorine gas at 3 5 0 K and 2 . 3 0 atm using
#a Calculate the molar volume of chlorine gas at and atm using the ideal gas law.
b Repeat part a but use the van der Waals equation of state. The van der Waals constants are atm and
Hint: try an iterative procedure, starting with the ideal gas volume as a first estimate for the attractive term. Do two iterations to reach the answer
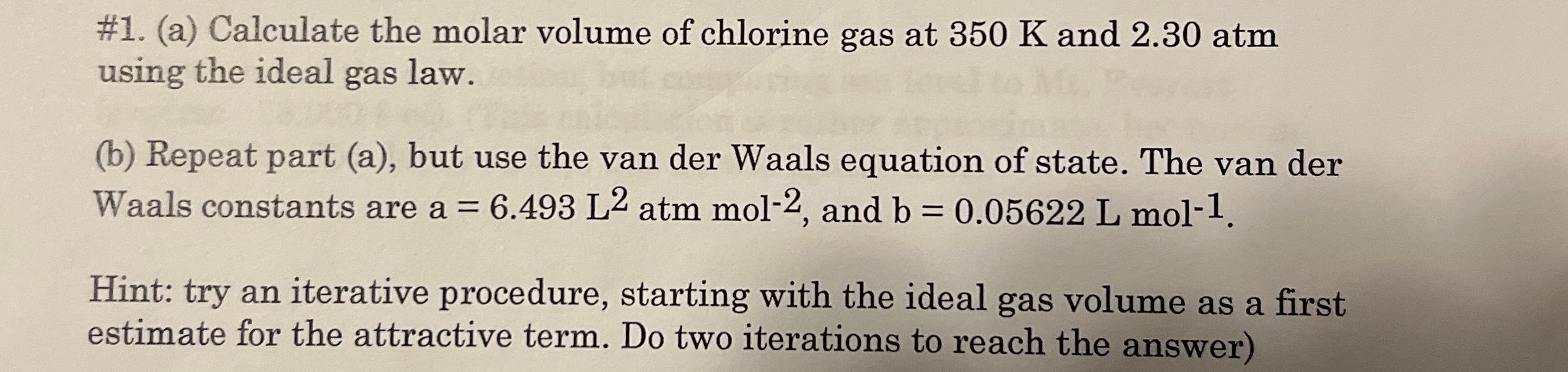
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


