Question: 1. A continuous distillation column is to be used to separate an aromatic/aliphatic mixture at a rate of 300kmolhr1. The mixture, which is a saturated
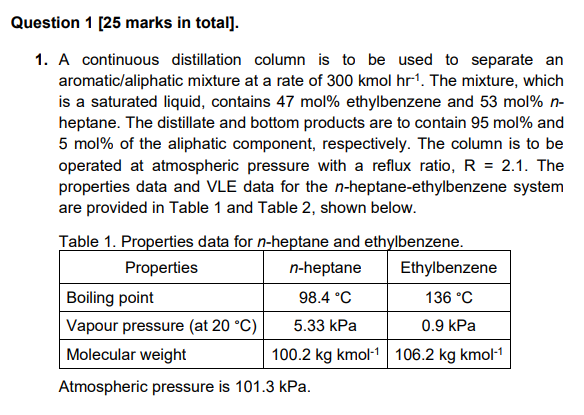
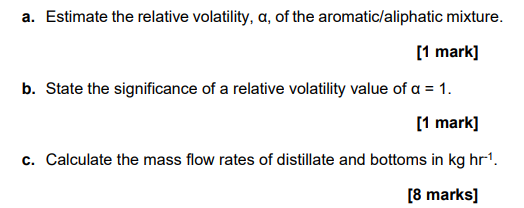

1. A continuous distillation column is to be used to separate an aromatic/aliphatic mixture at a rate of 300kmolhr1. The mixture, which is a saturated liquid, contains 47mol% ethylbenzene and 53mol%n heptane. The distillate and bottom products are to contain 95mol% and 5mol% of the aliphatic component, respectively. The column is to be operated at atmospheric pressure with a reflux ratio, R=2.1. The properties data and VLE data for the n-heptane-ethylbenzene system are provided in Table 1 and Table 2, shown below. Table 1. Properties data for n-heptane and ethylbenzene. Atmospheric pressure is 101.3kPa. a. Estimate the relative volatility, , of the aromatic/aliphatic mixture. [1 mark] b. State the significance of a relative volatility value of =1. [1 mark] c. Calculate the mass flow rates of distillate and bottoms in kghr1. [8 marks] d. Use the VLE data provided to plot the corresponding xy diagram of the system. Using the McCabe-Thiele graphical method, estimate the number of equilibrium stages in the distillation column and identify the location of the feed tray. Assume that a total condenser and partial reboiler are used. The q-line, enrichment section operating line (ESOL), stripping section CHEN20072 operating line (SSOL) and steps must be shown in the xy diagram plotted. Include your xy diagram with your answer sheet. [10 marks] e. If the feed condition changes to a saturated vapour and the reflux ratio remains the same, R=2.1, would more or fewer stages be required for the separation? Briefly explain why
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


