Question: 1) A dimerization reaction, 2A A2, occurs in a surface-catalyzed manner through the following kinetics: ki A+X = AX (R1) k-1 k2 AX + A-3
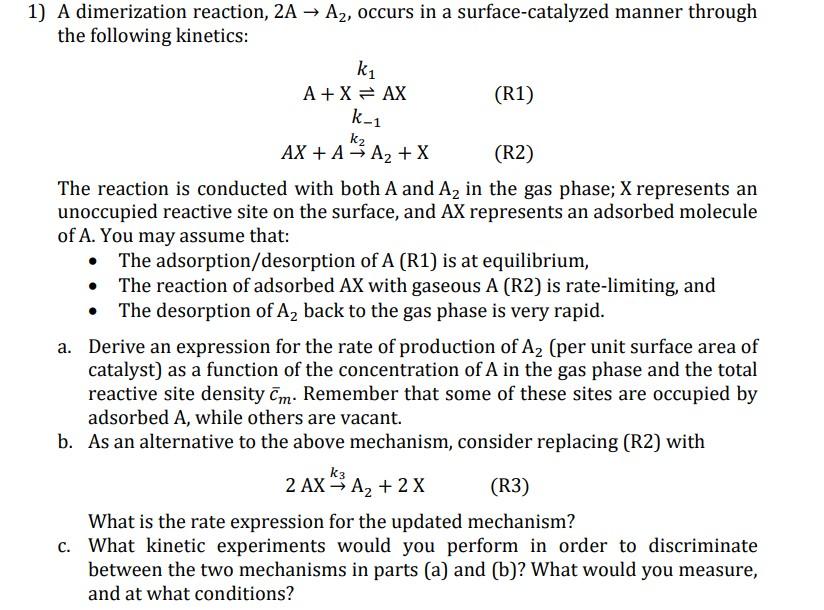
1) A dimerization reaction, 2A A2, occurs in a surface-catalyzed manner through the following kinetics: ki A+X = AX (R1) k-1 k2 AX + A-3 A2 + x (R2) The reaction is conducted with both A and A2 in the gas phase; X represents an unoccupied reactive site on the surface, and AX represents an adsorbed molecule of A. You may assume that: The adsorption/desorption of A (R1) is at equilibrium, The reaction of adsorbed AX with gaseous A (R2) is rate-limiting, and The desorption of Az back to the gas phase is very rapid. a. Derive an expression for the rate of production of Az (per unit surface area of catalyst) as a function of the concentration of A in the gas phase and the total reactive site density Cm. Remember that some of these sites are occupied by adsorbed A, while others are vacant. b. As an alternative to the above mechanism, consider replacing (R2) with k3 2 AX 3 A2 + 2x (R3) What is the rate expression for the updated mechanism? c. What kinetic experiments would you perform in order to discriminate between the two mechanisms in parts (a) and (b)? What would you measure, and at what conditions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


