Question: 1. A drug sample was found to decompose at 40 oC. If the observed rate constant = 1.7 x 10-3 hr-1 and the spontaneous rate
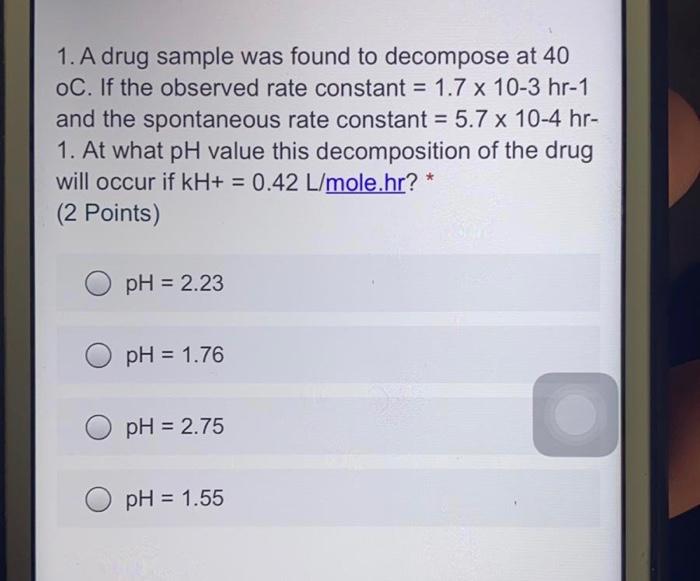
1. A drug sample was found to decompose at 40 oC. If the observed rate constant = 1.7 x 10-3 hr-1 and the spontaneous rate constant = 5.7 x 10-4 hr- 1. At what pH value this decomposition of the drug will occur if kH+ = 0.42 L/mole.hr? (2 Points) pH = 2.23 pH = 1.76 pH = 2.75 pH = 1.55
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


