Question: 1. A Hydrogen atom initially in its ground state i.e., n = 1 level, absorbs a photon and ends up in n= 4 level. What

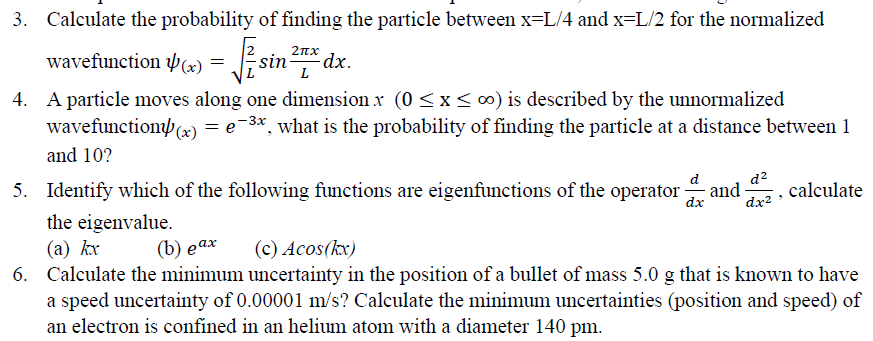
1. A Hydrogen atom initially in its ground state i.e., n = 1 level, absorbs a photon and ends up in n= 4 level. What must have been the frequency of the photon? Now the electron makes spontaneous emission and jumps to lower levels. What are the possible frequencies of the photons emitted during this process? Calculate the wavelength of the emitted light and specify which series are they in (Lyman series, Balmer series or Paschen series) = L 3. Calculate the probability of finding the particle between x=L/4 and x=L/2 for the normalized 21x wavefunction (x) = 4 sin dx. 4. A particle moves along one dimension x (0 SX500) is described by the unnormalized wavefunction)(x) = 2-3x, what is the probability of finding the particle at a distance between 1 and 10? 5. Identify which of the following functions are eigenfunctions of the operator and calculate the eigenvalue. (a) kx (c) Acos(kx) 6. Calculate the minimum uncertainty in the position of a bullet of mass 5.0 g that is known to have a speed uncertainty of 0.00001 m/s? Calculate the minimum uncertainties (position and speed) of an electron is confined in an helium atom with a diameter 140 pm. d dx d2 dx2 (b) eax
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


