Question: 1. a) Particle inside an infinite potential well problem in 3-D, gives as one of its result the following dependency between energy and volume Ex
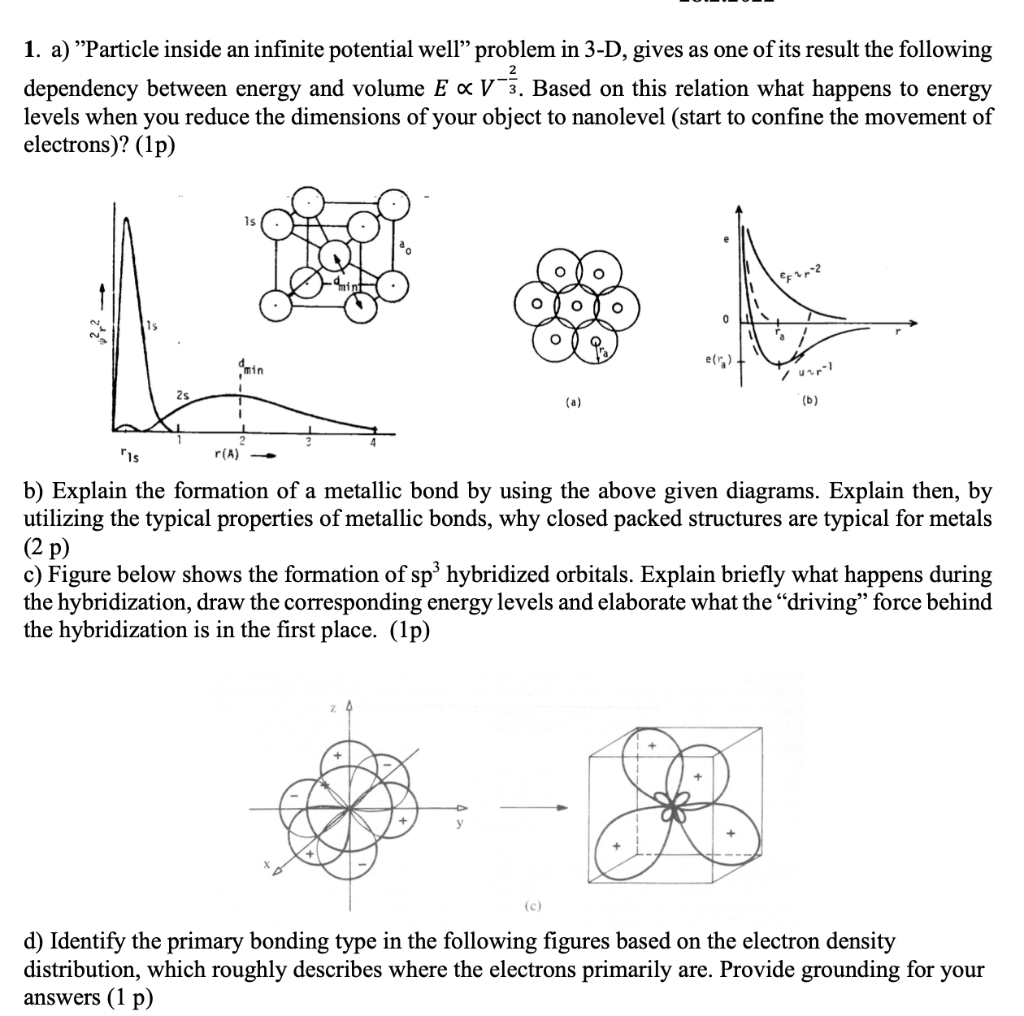
1. a) Particle inside an infinite potential well problem in 3-D, gives as one of its result the following dependency between energy and volume Ex V 3. Based on this relation what happens to energy levels when you reduce the dimensions of your object to nanolevel (start to confine the movement of electrons)? (1p) 1s CF22 0 min er Up 25 (a) (b) "is r(A) - b) Explain the formation of a metallic bond by using the above given diagrams. Explain then, by utilizing the typical properties of metallic bonds, why closed packed structures are typical for metals (2 p) c) Figure below shows the formation of sp hybridized orbitals. Explain briefly what happens during the hybridization, draw the corresponding energy levels and elaborate what the driving force behind the hybridization is in the first place. (1p) (c) d) Identify the primary bonding type in the following figures based on the electron density distribution, which roughly describes where the electrons primarily are. Provide grounding for your answers (1 p)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


