Question: 1. A product, P is to be produced from reactant R1 and reactant R2. In the first stage of the process, reactant Riis decomposed at

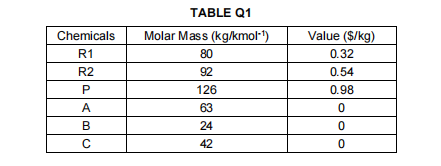
1. A product, P is to be produced from reactant R1 and reactant R2. In the first stage of the process, reactant Riis decomposed at 550C and 1.013 bar to intermediate A via the reaction: R1 + A+B Unfortunately, some of the intermediate A formed decomposes further into unwanted byproduct C via the reaction: AC Laboratory studies of these reactions indicate that the intermediate A selectivity, S varies with conversion, X which follows the relationship: S = 1 -0.6% The second stage of the reaction requires the intermediate A to be reacted with reactant Rzat 70C and 1.013 bar to produce the desired product P via the reaction: 2A + R2 + P The values of the chemicals involved, together with their molar masses are given in TABLE Q1. As an engineer, you are required to evaluate the economic potential of the plant with and without the presence of the side reaction. State all assumption and equations used. Chemicals R1 R2 TABLE Q1 Molar Mass (kg/kmol) 80 92 126 63 24 42 P A B Value ($/kg) 0.32 0.54 0.98 0 0 0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


