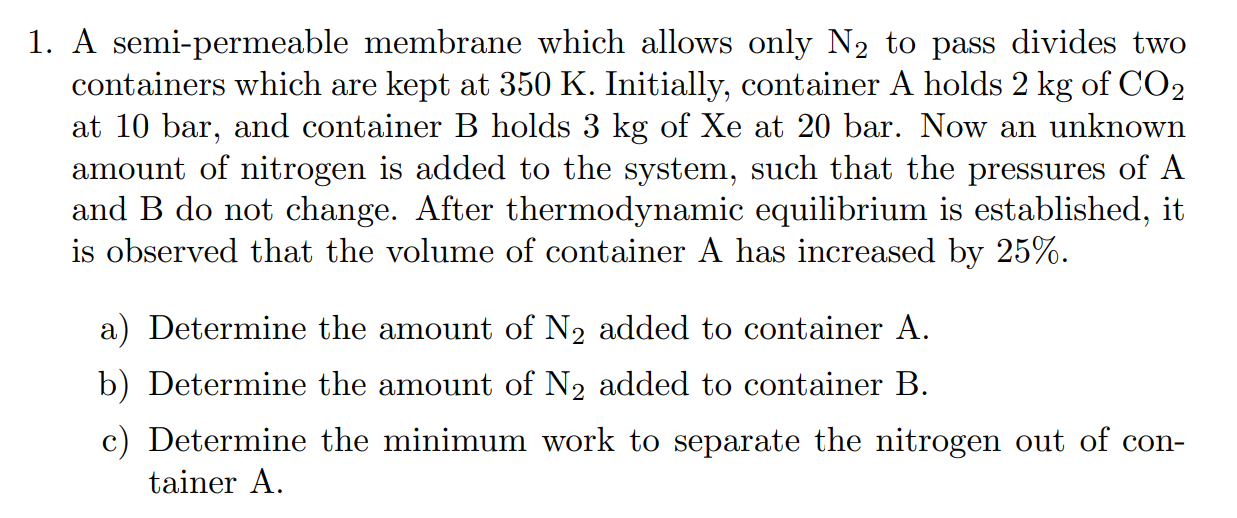
Question: 1 . A semi - permeable membrane which allows only N 2 to pass divides two containers which are kept at 3 5 0 K
A semipermeable membrane which allows only N to pass divides two containers which are kept at K Initially, container A holds kg of CO at bar, and container B holds kg of Xe at bar. Now an unknown amount of nitrogen is added to the system, such that the pressures of A and B do not change. After thermodynamic equilibrium is established, it is observed that the volume of container A has increased by a Determine the amount of N added to container A b Determine the amount of N added to container B c Determine the minimum work to separate the nitrogen out of container A A semipermeable membrane which allows only mathrmN to pass divides two containers which are kept at K Initially, container A holds kg of mathrmCO at bar, and container B holds kg of Xe at bar. Now an unknown amount of nitrogen is added to the system, such that the pressures of A and B do not change. After thermodynamic equilibrium is established, it is observed that the volume of container A has increased by
a Determine the amount of mathrmN added to container A
b Determine the amount of mathrmN added to container B
c Determine the minimum work to separate the nitrogen out of container A
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


