Question: 1 A work breakdown structure (an organization chart type preferred) Greensboro Chemical Products Corp. has done sufficient new product development at the research and development
1 A work breakdown structure (an organization chart type preferred)
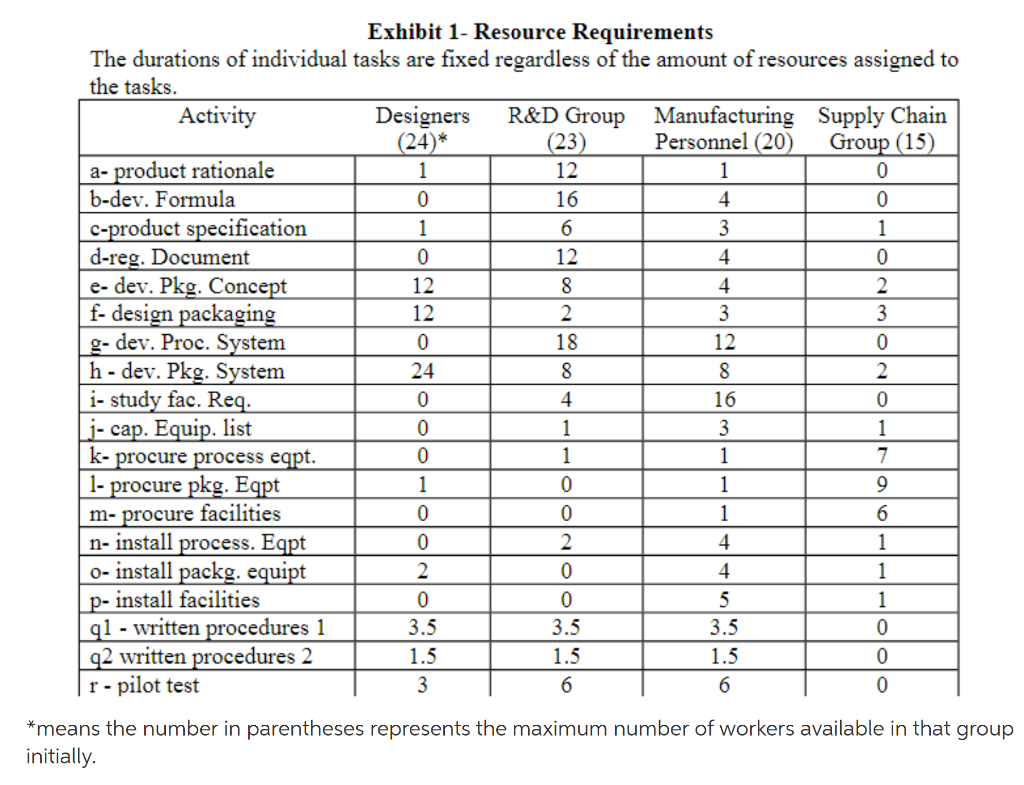
Greensboro Chemical Products Corp. has done sufficient new product development at the research and development level to estimate a high likelihood of technical success for a product of assured commercial success: A long-term antiseptic. Management has instructed Greensboro Chemicals Antiseptic Division to make a market entry at the earliest possible time; they have also requested a complete project plan up to the startup of production. Project responsibility is assigned to the divisions research and development group; Brandon Phillips, the project scientist who developed the product, is assigned responsibility for project management. Assistance will be required from other parts of the company: Design Engineers, R&D group, Manufacturing personnel, and Supply Chain group. Brandon as concerned about the scope of the project. He knew from his own experience that a final formula had yet to be developed, although such development was really a routine function. The remaining questions had to do with color, odor, and consistency additives rather than any performance-related modification. Fortunately, the major regulatory issues had also been resolved and he believed that submission of regulatory documentation would be followed by rapid approval as they already had a letter of approval contingent on final documentation. Brandon was concerned about defining the project unambiguously. To that end, he obtained an interview with Bud Miles, the group vice-president. When he asked Miles where his responsibility should end, the executive turned the question back to him. Brandon had been prepared for this and said that he would like to regard his part of the project as done when the production process could be turned over to manufacturing. They agreed that according to Ridges practice, this would be when the manufacturing operation could produce a 97% yield of product (fully packaged) at a level of 93% of the full production goal of 14 million liters per year. But I want you to remember, said Miles, that you must meet all current FDA, EPA, and OSHA regulations and you must be in compliance with our internal specification - the one I have is dated September and is RD15/918. And you know that manufacturing now - quite rightly, I feel - insists on fully documented procedures. After this discussion, Brandon felt that he had enough information about this aspect to start to pin down what had to be done to achieve these results. His first step in this effort was to meet with Rob Owens, the director of research. You are nave if you think that you can just start right in finalizing the formula, said Owens. You must first develop a product rationale (a). This is a formally defined process according to company policy. Marketing expects inputs at this stage, manufacturing expects its voice heard, and you will have to have approvals from every unit of the company that is involved. You should have no trouble if you do your homework, expect to spend a good eight weeks to get this done. That certainly stretches things out, said Brandon. I expected to take 12 weeks to develop the ingredient formula (b) and you know that I cant start to establish product specifications (c) until the formula is complete. Thats another 3 weeks. Yes, but while you are working on the product specifications you can get going on the regulatory documentation (d). Full internal specifications are not required for that work, but you cant start those documents until the formula is complete. Yes, and I find it hard to believe that we can push through both preparation of documents and getting approval in 3 weeks, but Environmental swears it can be done. Oh, it can be done in this case because of the preparatory work. Of course, I wont say that this estimate of 3 weeks is as certain as our other estimates. All we need is a change of staff at the Agency and we are in trouble. But once you have both the specifications, and the approval, you can immediately start on developing the processing system (g). "Yes, and how I wish we could get a lead on that, but the designers say that there is too much uncertainty and they won't move until they have both specifications and regulatory documentation and approval. They are offering pretty fast response; six weeks from start to finish for the processing system." "They are a good crew, Brandon. And of course, you know that you don't have to delay on starting the packaging segment of this project. You can start developing the packaging concept (e) just as soon as the product rationale has been developed. If my experience is any judge, it will take a full eight weeks; you'll have to work to keep the process from running forever." "But as soon as that is finished we can start on the design of the package and its materials (f) which usually takes about six weeks. Once that is done we can start on the packaging system (h) which shouldn't take longer than eight weeks," concluded Brandon. At this point he realized that although Owens would have general knowledge, he needed to talk directly to the Director of Manufacturing. "The first step, which follows the completion of the development of processing and packaging systems," said the Director of Manufacturing, "is to do a complete study of the facilities requirements (i). You won't be able to get that done in less than four weeks. And that must precede the preparation of the capital equipment list (j) which should take about three- quarters as long. Of course, as soon as both the process system and packaging system are completed, you could start on preparing the written manufacturing procedures (q)." "But, said Brandon, "Can I really finish the procedures before I have installed and constructed the facilities (p)?" "No, quite right. What you can do is get the first phase done, but the last phase will have to wait for the installation and construction." "Then this means that I really have two phases for the writing, that which can be completed without the installation and construction (q1), which will take seven weeks, and that which has to wait for those inputs (q2) which will require 3 weeks." "True. Now you realize that the last thing you have to do is to run the equipment in a pilot test (r) which will show that you have reached a satisfactory level?" "Yes. Since that must include debugging, I've estimated a six-week period as adequate." The director of manufacturing assented. Brandon continued, "What I'm not sure of is whether we can run all the installation tasks in parallel." "You can get the purchase orders and carry out the procurement of process equipment (k), packaging equipment (l), and facilities (m) as soon as the capital equipment list is complete. The installation of each of these types of equipment and facilities can start as soon as the goods are on hand (n,o,p)". "What do you estimate for the times to do these tasks?" asked Brandon. The director of manufacturing estimated 18, 8, and 4 weeks for the purchasing phases for each of the subsystems in that order and four weeks for each of the installations. "Then I can regard my job as done with the delivery of the procedures and when I show my 96 percent yield," said Brandon, and the director of manufacturing agreed, but reminded Brandon that none of the purchasing cycles could start until the capital equipment list had been prepared and approved (j) which he saw as a three-week task. The executive committee of Ridge Chemical Products Corporation set a starting date for the project of June 4, 2018 and asked Brandon to project a completion date with his submission of the plan. The committee's request implied that whatever date Brandon came up with was acceptable, but Brandon knew that he would be expected to show how to shorten the time to complete the project. However, his task in making the schedule was clear; he had to establish the resource requirements and deal with calendar constraints as best as he could. To this end, Brandon had to get an estimate of resources which he decided to do by making a list of the activities and asking each group involved what was their level of employee input. The results of this survey are shown in Exhibit 1.
 A work breakdown structure (an organization chart type preferred)
A work breakdown structure (an organization chart type preferred)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


