Question: Given the following information: nitrous acid aniline. HNO CH;NH, K=4.5x104 Kb = 7.410-10 (1) Write the net ionic equation for the reaction that occurs
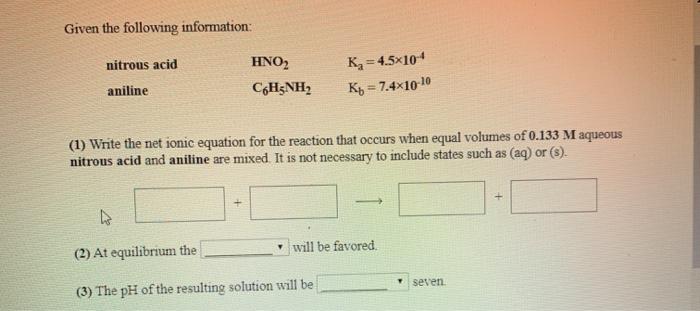
Given the following information: nitrous acid aniline. HNO CH;NH, K=4.5x104 Kb = 7.410-10 (1) Write the net ionic equation for the reaction that occurs when equal volumes of 0.133 M aqueous nitrous acid and aniline are mixed. It is not necessary to include states such as (aq) or (s). 4 (2) At equilibrium the (3) The pH of the resulting solution will be will be favored. seven.
Step by Step Solution
★★★★★
3.44 Rating (173 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

1 HNO2 K Cam PH sight ... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


