Question: 1) (application) C02 (shown below) is a linear molecule and is not polar (i.e. there is no clear negatively charged side or positively charged side).
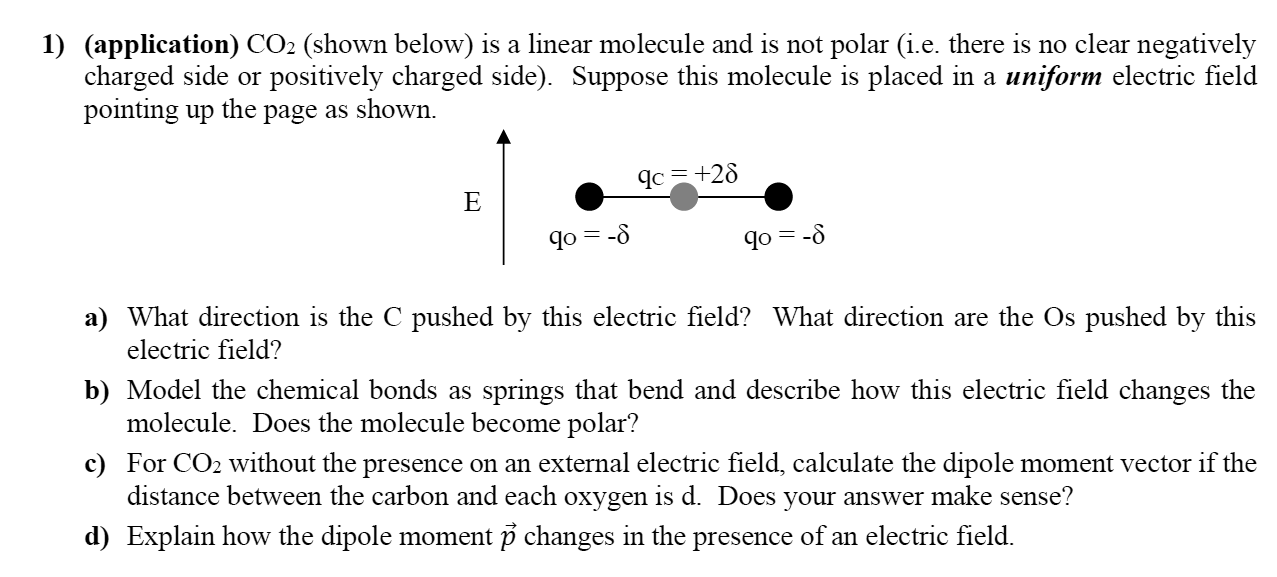
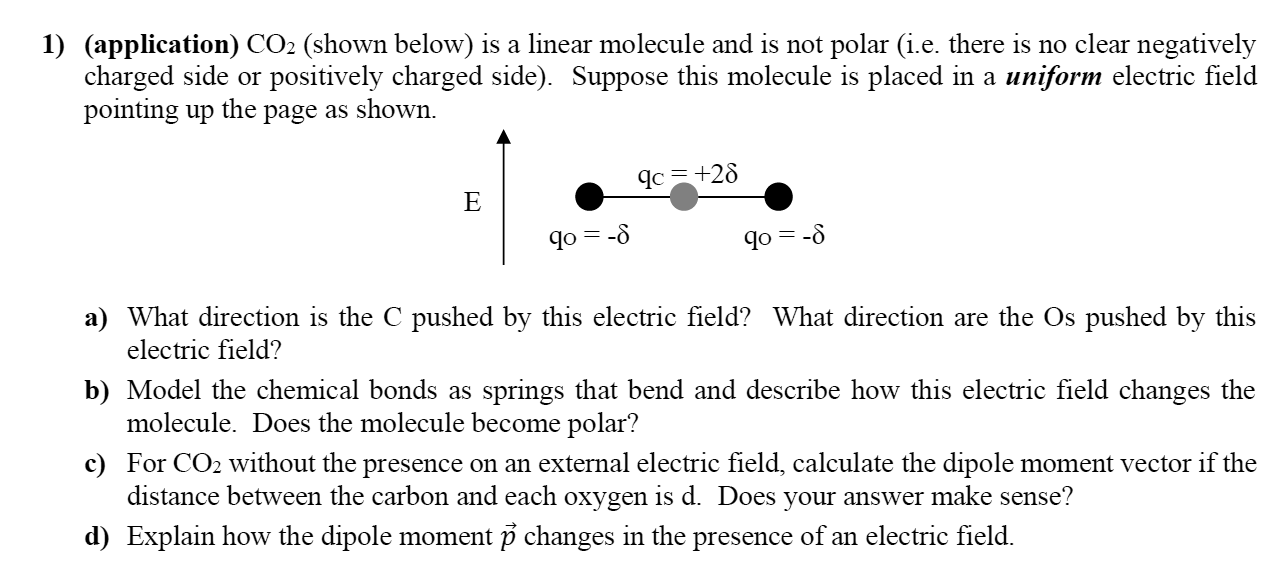
1) (application) C02 (shown below) is a linear molecule and is not polar (i.e. there is no clear negatively charged side or positively charged side). Suppose this molecule is placed in a uniform electric. eld pointing up the page as shown. (IC = +25 E 000 qo:5 qo:-5 a) What direction is the C pushed by this electric eld? What direction are the Os pushed by this electric field? b) Model the chemical bonds as springs that bend and describe how this electric field changes the molecule. Does the molecule become polar? c) For C 02 without the presence on an external electric eld, calculate the dipole moment vector if the distance between the carbon and each oxygen is d. Does your answer make sense? (1) Explain how the dipole moment 13 changes in the presence of an electric field
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


