Question: 1- At 303K, the vapor pressure of benzene is 118 Torr and that of cyclohexane is 122 Torr. Calculate the vapor pressure of a solution

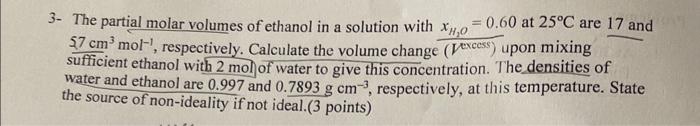
1- At 303K, the vapor pressure of benzene is 118 Torr and that of cyclohexane is 122 Torr. Calculate the vapor pressure of a solution for which xberzene=0.25 assuming ideal behavior. ( 2 points) Ptis122Pbene=118xb1+20=0.25xcydo=10Ls 2- An ideal solution is formed by mixing liquids A and B at 298K. The vapor pressure of pure A is 180 Torr and that of pure B is 82.1 Torr. If the mole fraction of A in the vapor is 0.450, what is the mole fraction of A in the solution? (3 points) 3- The partial molar volumes of ethanol in a solution with xH2O=0.60 at 25C are 17 and 57cm3mol1, respectively. Calculate the volume change ( Vexcess) upon mixing sufficient ethanol with 2mol of water to give this concentration. The densities of water and ethanol are 0.997 and 0.7893gcm3, respectively, at this temperature. State the source of non-ideality if not ideal.(3 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


