Question: 1 b Water with partial analysis results shown in Table 1 is to be softened by the excesslime-soda ash method. Determine the amount of each
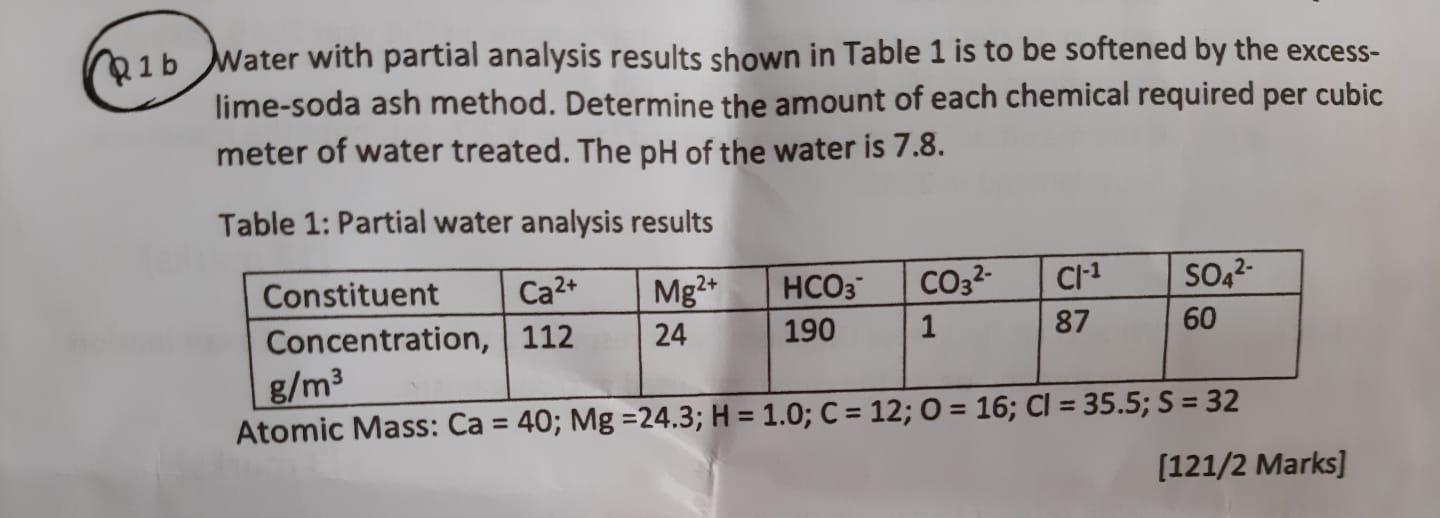
1 b Water with partial analysis results shown in Table 1 is to be softened by the excesslime-soda ash method. Determine the amount of each chemical required per cubic meter of water treated. The pH of the water is 7.8. Table 1: Partial water analysis results Atomic Mass: Ca=40;Mg=24.3;H=1.0;C=12;O=16;Cl=30.0;0=02 [121/2 Marks]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


