Question: 1. Calculated for the following reactions. A+2 B3C+D 2A + 3 B 4C +2 D 2. A+ 3B = 2C + D, if we know
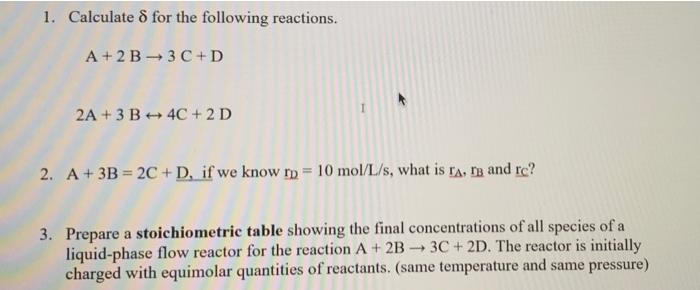
1. Calculated for the following reactions. A+2 B3C+D 2A + 3 B 4C +2 D 2. A+ 3B = 2C + D, if we know rp = 10 mol/L/s, what is ra, ng and re? a 3. Prepare a stoichiometric table showing the final concentrations of all species of a liquid-phase flow reactor for the reaction A + 2B 3C + 2D. The reactor is initially charged with equimolar quantities of reactants. (same temperature and same pressure)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


