Question: 1. Chapter 6 - A jar test run with ferric chloride (FeCl3) as the coagulant in a surface water (pH = 7.5), results in an
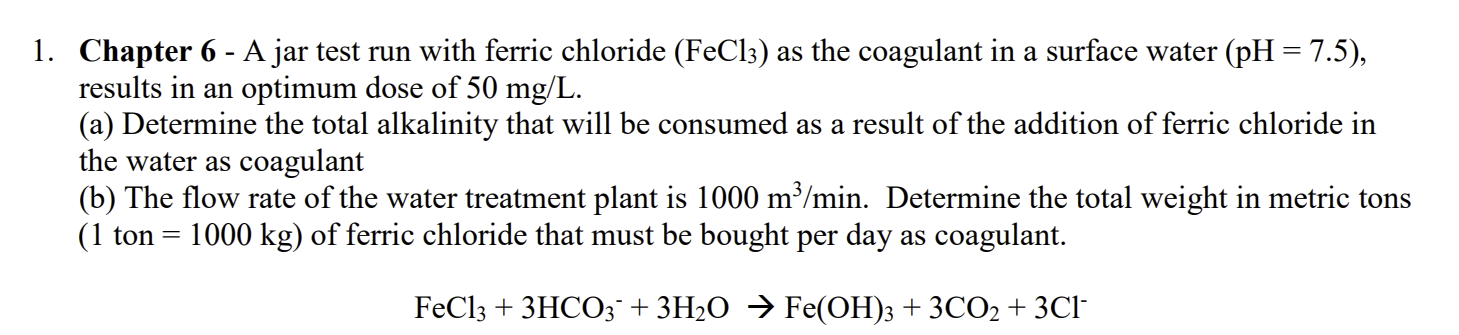
1. Chapter 6 - A jar test run with ferric chloride (FeCl3) as the coagulant in a surface water (pH = 7.5), results in an optimum dose of 50 mg/L. (a) Determine the total alkalinity that will be consumed as a result of the addition of ferric chloride in the water as coagulant (b) The flow rate of the water treatment plant is 1000 m3/min. Determine the total weight in metric tons (1 ton = 1000 kg) of ferric chloride that must be bought per day as coagulant. = FeCl3 + 3HCO3 + 3H2O Fe(OH)3 + 3CO2 + 3Cl
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


