Question: 1. Complete the following equilibrium problems. a. Consider the following information: 2A(g)+3B(g)=2D(g)2D(g)+2B(g)=2E(g)Keq=1.2103Keq=4.4105 Use the information above to calculate the value of Keq for the following
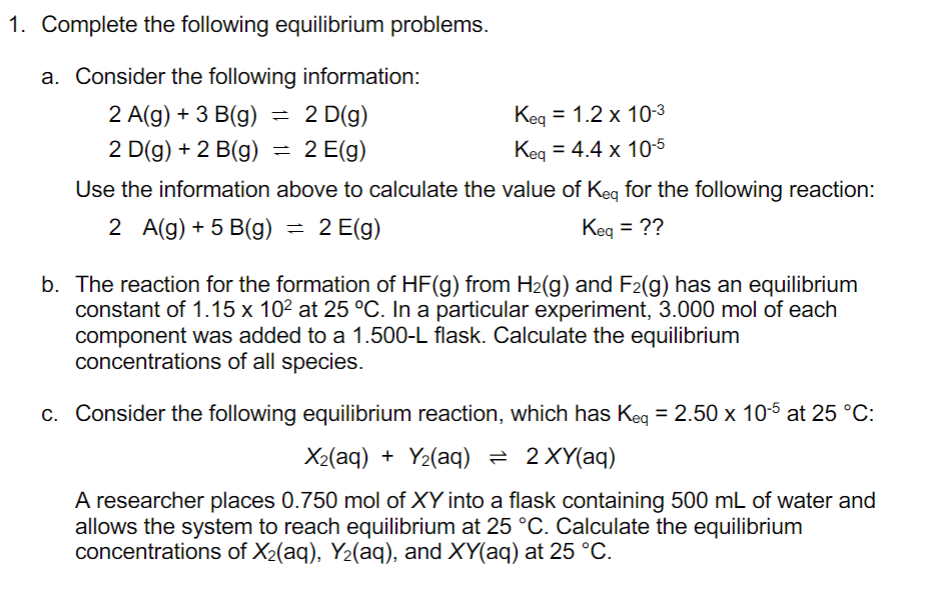
1. Complete the following equilibrium problems. a. Consider the following information: 2A(g)+3B(g)=2D(g)2D(g)+2B(g)=2E(g)Keq=1.2103Keq=4.4105 Use the information above to calculate the value of Keq for the following reaction: 2A(g)+5B(g)=2E(g)Keq=?? b. The reaction for the formation of HF(g) from H2(g) and F2(g) has an equilibrium constant of 1.15102 at 25C. In a particular experiment, 3.000mol of each component was added to a 1.500-L flask. Calculate the equilibrium concentrations of all species. c. Consider the following equilibrium reaction, which has Keq=2.50105 at 25C : X2(aq)+Y2(aq)2XY(aq) A researcher places 0.750mol of XY into a flask containing 500mL of water and allows the system to reach equilibrium at 25C. Calculate the equilibrium concentrations of X2(aq),Y2(aq), and XY(aq) at 25C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


