Question: 1) Complete the following table based on the experiments you did in the lab: (4marks) begin{tabular}{|l|l|l|l|} hline Experiment & Quantity measured & Quantity determined &
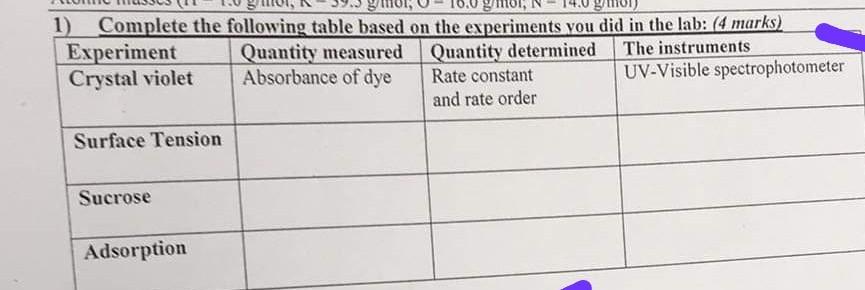

1) Complete the following table based on the experiments you did in the lab: (4marks) \begin{tabular}{|l|l|l|l|} \hline Experiment & Quantity measured & Quantity determined & The instruments \\ \hline Crystal violet & Absorbance of dye & Rate constant and rate order & UV-Visible spectrophotometer \\ \hline Surface Tension & & & \\ \hline Sucrose & & & \\ \hline Adsorption & & & \\ \hline \end{tabular} Q6) for the reaction of 2F+S2Os2l+2SO42 16.60g Potassium lodide (166.0g/mol) is dissolved in 250.00Vol. flask. 6.75g Potassium persulfate (270.0g/mol) is dissolved in 125.00mL Vol. flask. The components are mixed and after around five minutes, 10.0mL is taken to titrate with 0.020M thiosulfate solution according to the table below: a) Calculate the rate constant from the above experiment b) What is the of the volume of KCl(0.1M) that must be added to the above solution to make the rate constant equals to 0.45min1 (suppose the total volume does not change from the above experiment)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


