Question: 1. Complete the following table. Predict the chemical formula for the ionic compounds formed between each cation- anion pair. Na Mg AP Pb 0-
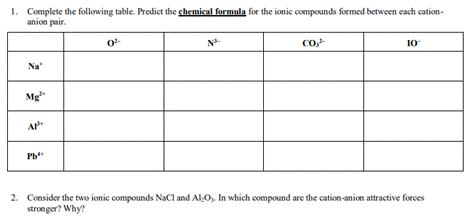
1. Complete the following table. Predict the chemical formula for the ionic compounds formed between each cation- anion pair. Na Mg AP Pb" 0- NA Co, 10 2. Consider the two ionic compounds NaCl and AlO,. In which compound are the cation-anion attractive forces stronger? Why?
Step by Step Solution
★★★★★
3.37 Rating (166 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

O 2 N 3 CO3 3 IO Na Na 2 O Na 3 N Na 2 CO 3 NaI Mg 2 2M... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


