Question: 1. Consider a batch reactor with two sequential reactions. The first reaction is a reversible reaction, A + B, while the second reaction is irreversible,
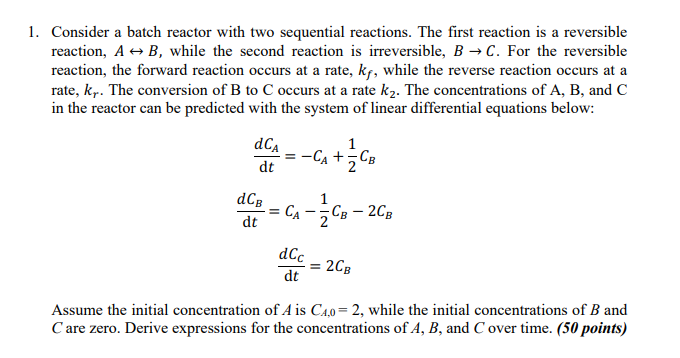
1. Consider a batch reactor with two sequential reactions. The first reaction is a reversible reaction, A + B, while the second reaction is irreversible, B C. For the reversible reaction, the forward reaction occurs at a rate, kf, while the reverse reaction occurs at a rate, kr. The conversion of B to Coccurs at a rate kz. The concentrations of A, B, and C in the reactor can be predicted with the system of linear differential equations below: 1 CA dt = -CA+zCb c dCB CA CB - 2CB dt dCc = 2CB dt Assume the initial concentration of Ais C4,0 = 2, while the initial concentrations of B and Care zero. Derive expressions for the concentrations of A, B, and Cover time. (50 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


