Question: 1. Consider pentane, CsH2 and 2,2-dimethylpropane, C(CH3). Predict which will have the higher melting point/boiling point. Explain your answer. 2 Marks/ 2. State what you

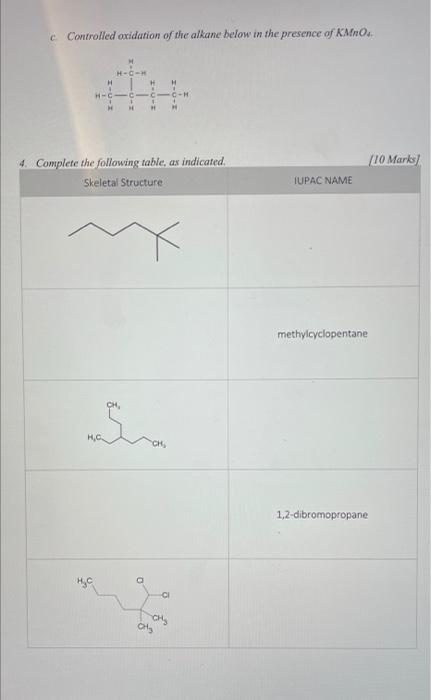
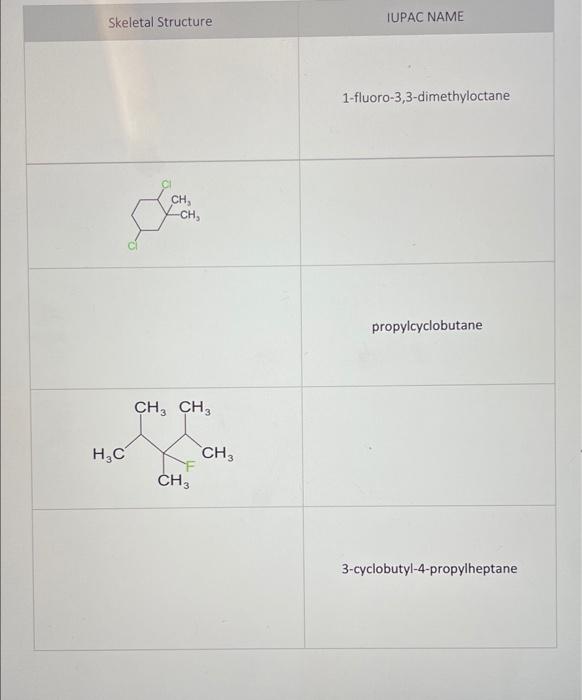
1. Consider pentane, CsH2 and 2,2-dimethylpropane, C(CH3). Predict which will have the higher melting point/boiling point. Explain your answer. 2 Marks/ 2. State what you expect to happen when you add I mL hexane to 1 mL water. Explain. [2 marks] [6 Marks] 3. Write balanced chemical equations for the following chemical reactions: a. Complete combustion of octane. b. Chlorination of the following alkane. Show all possible products and indicate which alkyl halide will be the most favored. H H-C-H H H H H- CCC -- C-H hw, A + Clas) H H H H Controlled oxidation of the alkane below in the presence of Mino H- H H- CC-H 1 1 HE (10 Marks 4. Complete the following table, as indicated. Skeletal Structure IUPAC NAME methylcyclopentane CH Si HC 1,2-dibromopropane CH ots Skeletal Structure IUPAC NAME 1-fluoro-3,3-dimethyloctane CH YCH, propylcyclobutane CH, CH, . CH CH3 3-cyclobutyl-4-propylheptane
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


