Question: 1) Consider the following acid-base reaction: H H a) Complete the reaction by drawing the products. Be sure to include formal charges and lone pairs
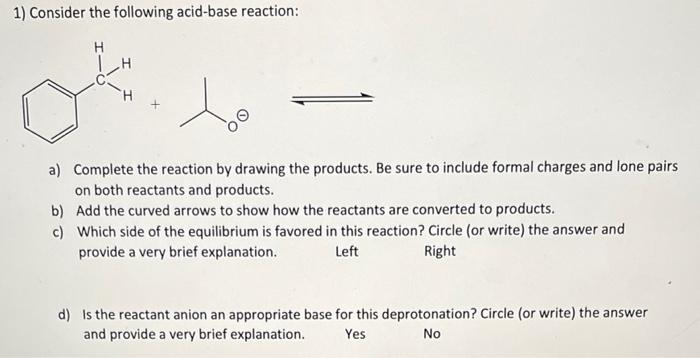
1) Consider the following acid-base reaction: H H a) Complete the reaction by drawing the products. Be sure to include formal charges and lone pairs on both reactants and products. b) Add the curved arrows to show how the reactants are converted to products. c) Which side of the equilibrium is favored in this reaction? Circle (or write) the answer and provide a very brief explanation. Left Right d) is the reactant anion an appropriate base for this deprotonation? Circle (or write) the answer and provide a very brief explanation. Yes No
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


