Question: 1. Consider the Lewis structures shown below and answer the following questions: a. Show all non-zero formal charges on your structures. b. Compare structures A,B
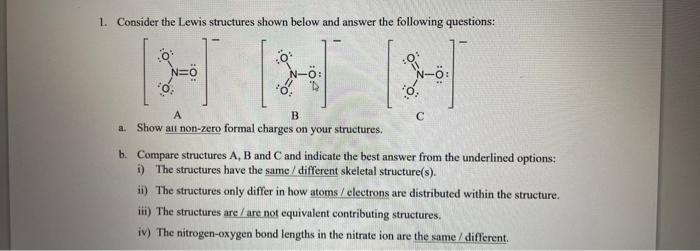
1. Consider the Lewis structures shown below and answer the following questions: a. Show all non-zero formal charges on your structures. b. Compare structures A,B and C and indicate the best answer from the underlined options: i) The structures have the same/different skeletal structure(s). ii) The structures only differ in how atoms / electrons are distributed within the structure. iii) The structures are / are not equivalent contributing structures. iv) The nitrogen-oxygen bond lengths in the nitrate ion are the same / different
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


