Question: 1) Consider the two structures below and answer all the questions: Figure Q1 a) Given the bond enthalpies in the table below and the value
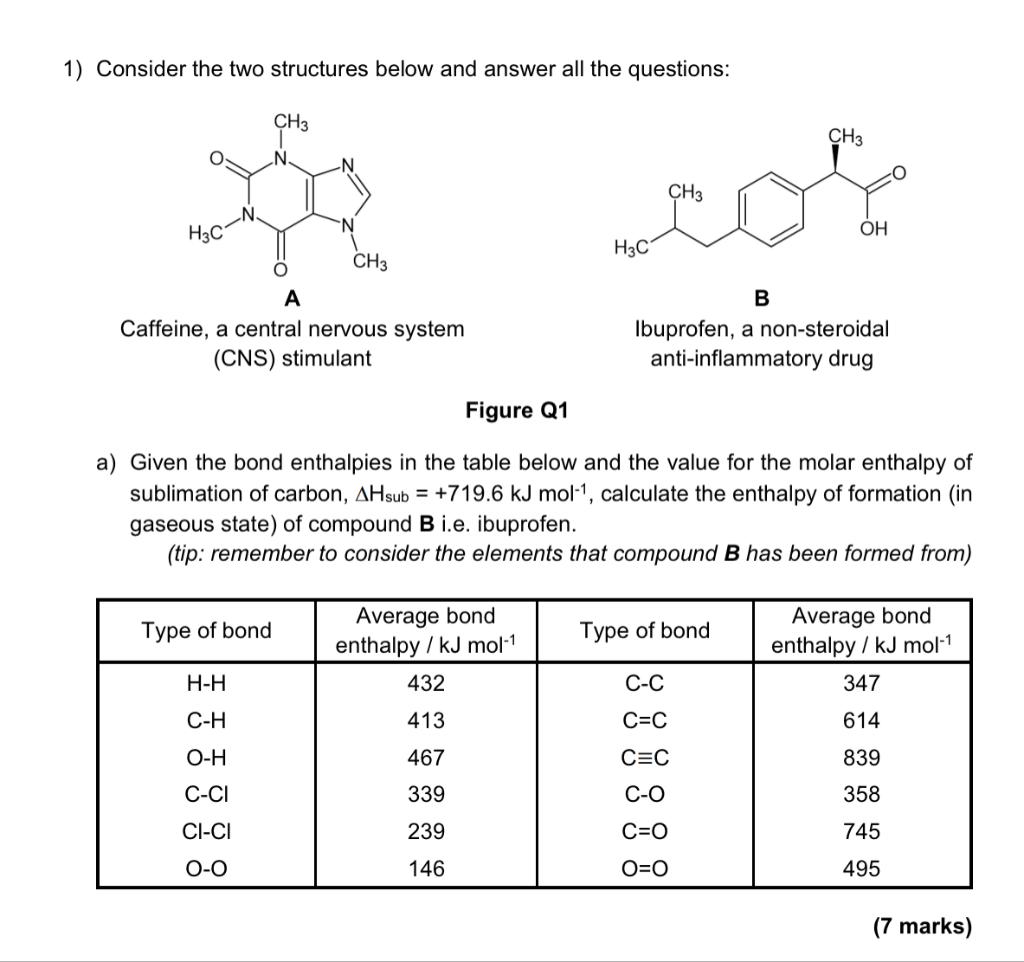
1) Consider the two structures below and answer all the questions: Figure Q1 a) Given the bond enthalpies in the table below and the value for the molar enthalpy of sublimation of carbon, Hsub=+719.6kJmol1, calculate the enthalpy of formation (in gaseous state) of compound B i.e. ibuprofen. (tip: remember to consider the elements that compound B has been formed from) (7 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


