Question: 1. For bcc structure a) Calculate the lattice parameter in terms of atomic radius. b) Calculate unit cell volume. c) What is the coordination number?
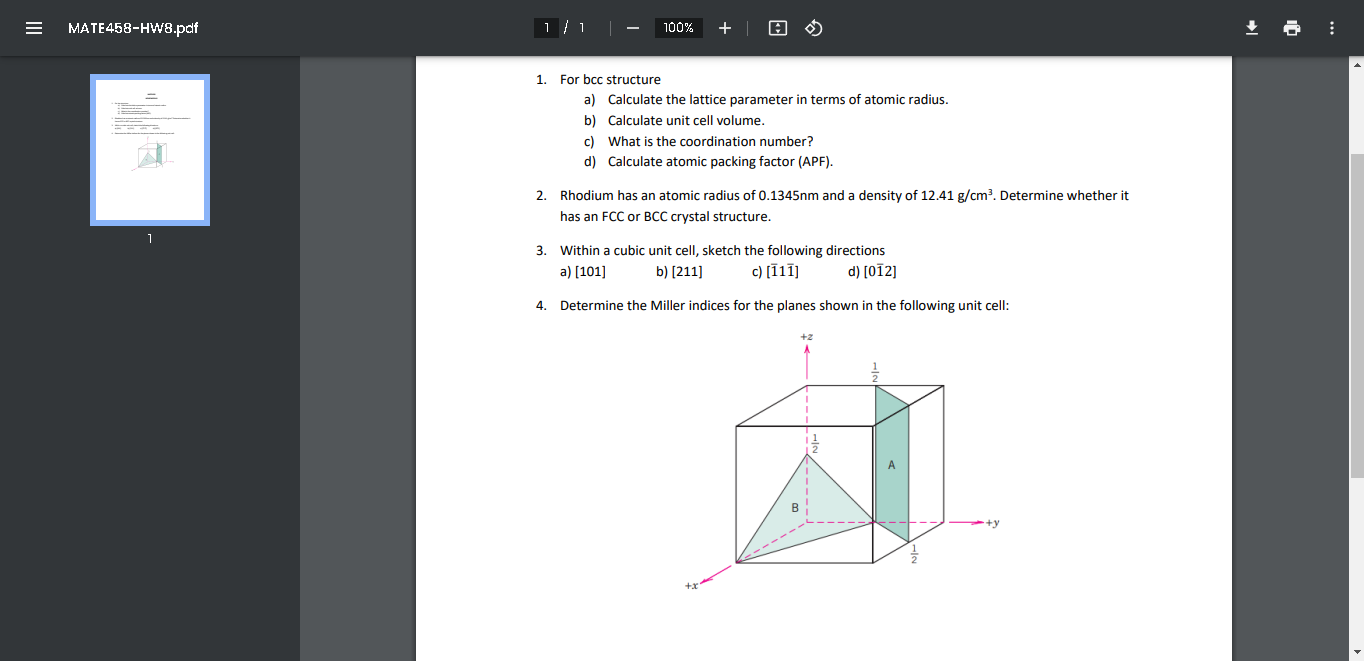
1. For bcc structure a) Calculate the lattice parameter in terms of atomic radius. b) Calculate unit cell volume. c) What is the coordination number? d) Calculate atomic packing factor (APF). 2. Rhodium has an atomic radius of 0.1345nm and a density of 12.41g/cm3. Determine whether it has an FCC or BCC crystal structure. 3. Within a cubic unit cell, sketch the following directions a) [101] b) [211] c) [111] d) [012] 4. Determine the Miller indices for the planes shown in the following unit cell
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


