Question: 1. Given the general esterification equation below. (3 Marks) RCOOH + R'OH RCOOR' + H20 a) Choose the best option(s) that would increase the formation

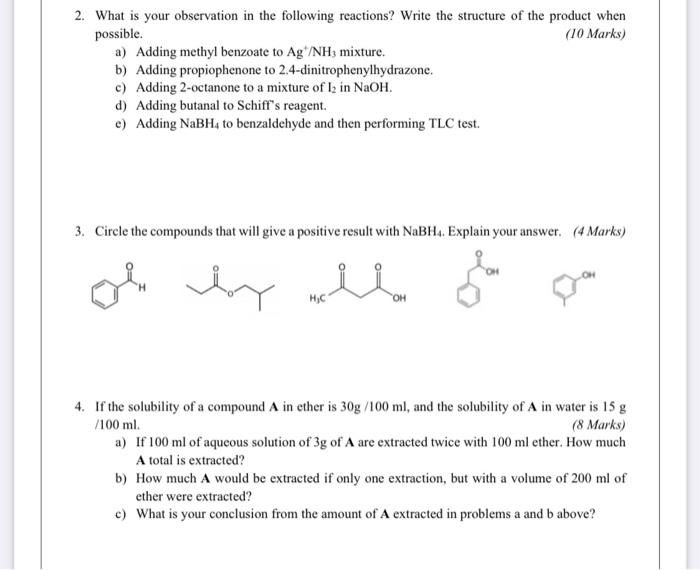
1. Given the general esterification equation below. (3 Marks) RCOOH + R'OH RCOOR' + H20 a) Choose the best option(s) that would increase the formation of the ester. Add excess of carboxylic acid Add excess of alcohol add excess water Remove water run the reaction in water as a solvent 2. What is your observation in the following reactions? Write the structure of the product when possible. (10 Marks) a) Adding methyl benzoate to Ag /NH, mixture. 2. What is your observation in the following reactions? Write the structure of the product when possible. (10 Marks) a) Adding methyl benzoate to Ag/NH3 mixture. b) Adding propiophenone to 2.4-dinitrophenylhydrazone. c) Adding 2-octanone to a mixture of lz in NaOH. d) Adding butanal to Schiff's reagent. c) Adding NaBH4 to benzaldehyde and then performing TLC test. 3. Circle the compounds that will give a positive result with NaBH. Explain your answer. (4 Marks) "OH 4. If the solubility of a compound A in ether is 30g/100 ml, and the solubility of A in water is 15 g /100 ml. (8 Marks) a) If 100 ml of aqueous solution of 3g of A are extracted twice with 100 ml ether. How much A total is extracted? b) How much A would be extracted if only one extraction, but with a volume of 200 ml of ether were extracted? c) What is your conclusion from the amount of A extracted in problems a and b above
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


