Question: 1. hexane (C 6 H 14 , H) and pentane (C 5 H 12 , P) are separated in a distillation column. In this distillation
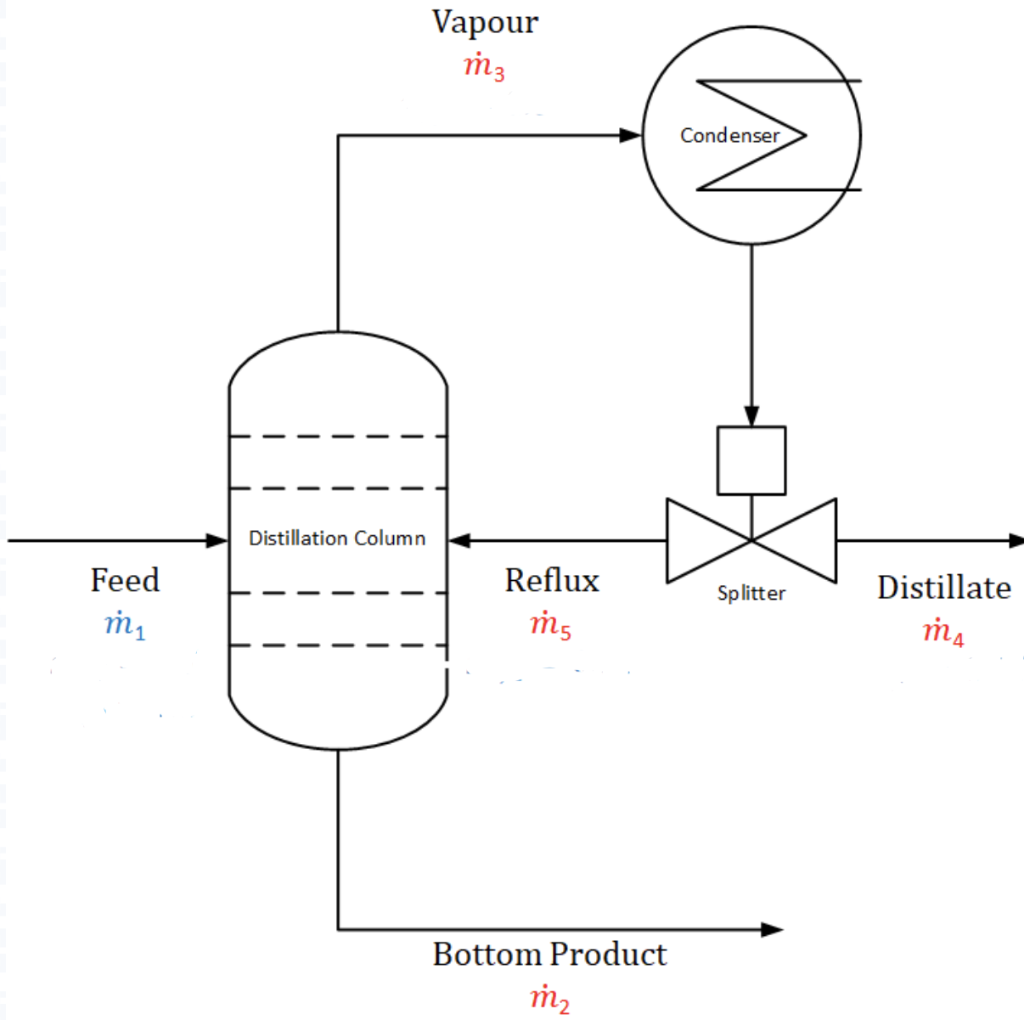
1. hexane (C6H14, H) and pentane (C5H12, P) are separated in a distillation column. In this distillation process, some of the vapour is returned to the distillation column after condensation to control the temperature and solute concentration. The ratio of this recycle sub-stream to the top product is called reflux ratio (reflux ratio = Reflux/Distillate = R/D). The mass flow rate of the feed stream is 1 = 840 kg/h, the feed contains 50 wt.% hexane (x1,H = 0.5), the distillate is 5 wt.% hexane (x4,H = 0.05), the bottom stream is 5 wt.% pentane (x2,P = 0.05), and reflux ratio is 0.60 (5 / 4 = 0.6).
The atomic masses are: H2 = 2 g/mol and C = 12 g/mol.
The densities are: hexane = 655 kg/m and pentane = 626 kg/m
a) What is the mass flow rate of the m2
b)What is the mass flow rate of the reflux stream
c) What is the mass flow rate of the vapour stream
Vapour m3 M. Condenser - - Distillation Column Feed m1 Distillate Reflux Splitter M4 Bottom Product m2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


