Question: 1 Hint 20 points 1 attempts left Check my work Be sure to answer all parts. Ethylene glycol (CH (OH)CH (OH)) is a common automobile
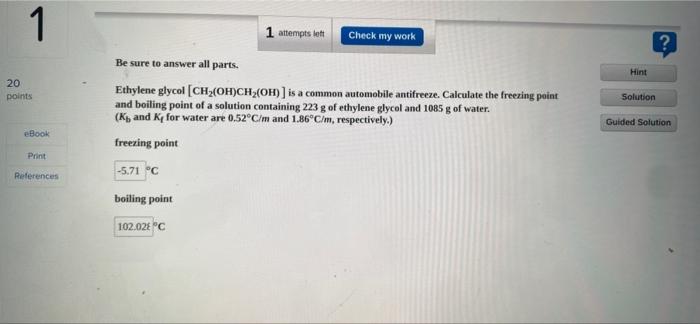
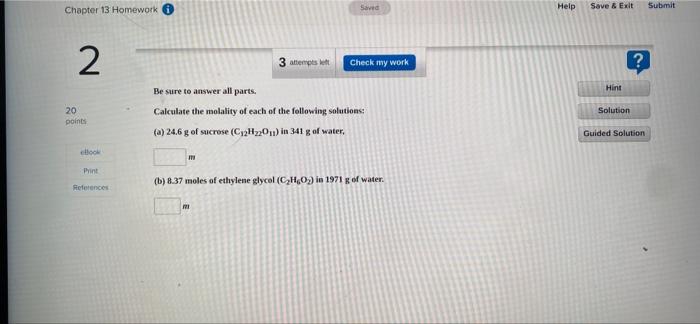
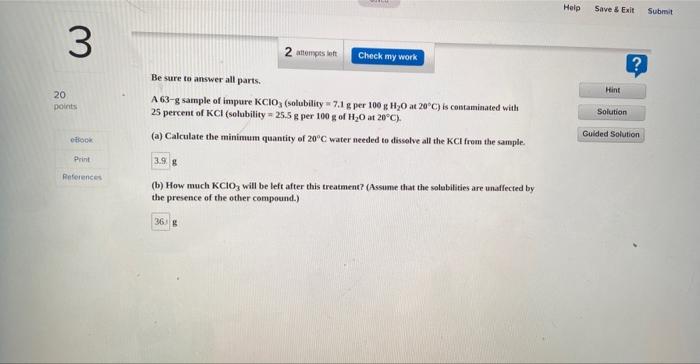
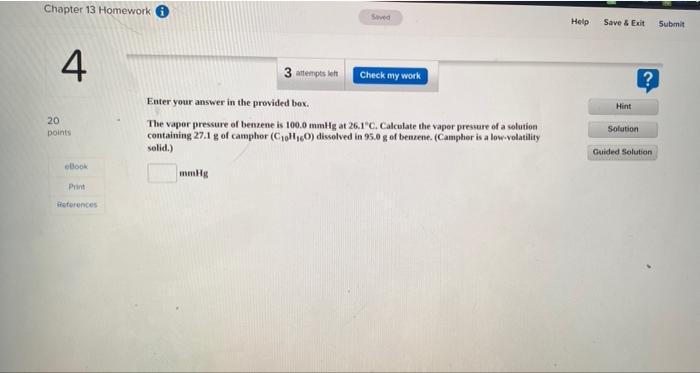
1 Hint 20 points 1 attempts left Check my work Be sure to answer all parts. Ethylene glycol (CH (OH)CH (OH)) is a common automobile antifreeze. Calculate the freezing point and boiling point of a solution containing 223 g of ethylene glycol and 1085 g of water. (Ky and Ky for water are 0.52C/m and 1.86C/m, respectively.) freezing point Solution Guided Solution eBook Print -5.71 C References boiling point 102.02 "C Saved Chapter 13 Homework Help Submit Save & Exit 2 3 attempts to Check my work ? Hint Be sure to answer all parts, Calculate the molality of each of the following solutions (a) 24.6 g of sucrose (C2H20.) in 341 g of water, 20 points Solution Guided Solution book IT Print (b) 8.37 moles of ethylene glycol (C,H,O) in 1971 g of water. References Help Save & Exit Submit 3 L? 2 attempts et Check my work Be sure to answer all parts. A63-g sample of impure KCIO(solubility 7.1 g per 100g H20 at 20C) is contaminated with 25 percent of KCl (solubility = 25.5 g per 100g of H20 at 20C). (a) Calculate the minimum quantity of 20C water needed to dissolve all the KCl from the sample. 20 points Hint Solution Guided Solution Print 3.98 References (b) How much KClo, will be left after this treatment? (Assume that the solubilities are unaffected by the presence of the other compound.) 36 Chapter 13 Homework Sved Help Save & Exit Submit 4 ? 3 attempts on Check my work Enter your answer in the provided box. The vapor pressure of bentene is 100.0 mmHg at 26.1"C. Calculate the vapor pressure of a solution containing 27.1 g of camphor (Ch.co) dissolved in 95.0 g of benzene. (Campher is a low volatility solid.) Hint 20 points Solution Guided Solution Book mmHg Print Roteroncos
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


