Question: 1. How many inner, outer, and valence electrons are present in an atom of each of the following elements? (a) O (b) Sn (c)
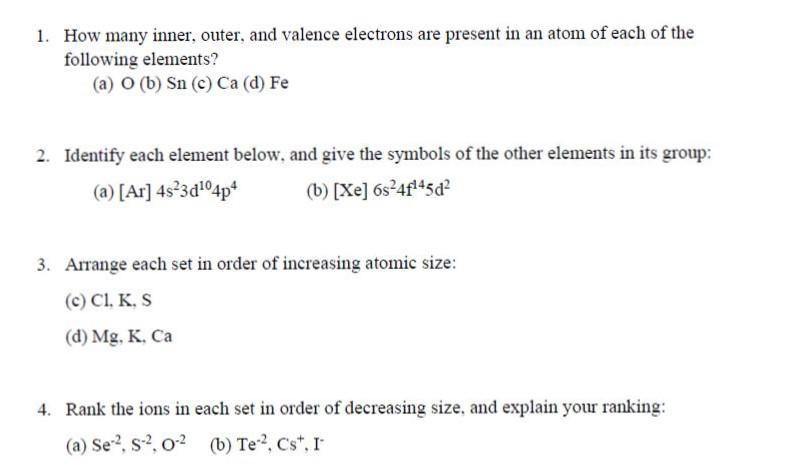
1. How many inner, outer, and valence electrons are present in an atom of each of the following elements? (a) O (b) Sn (c) Ca (d) Fe 2. Identify each element below, and give the symbols of the other elements in its group: (a) [Ar] 4s3d04p* (b) [Xe] 6s4f45d? 3. Arrange each set in order of increasing atomic size: (c) Cl, K, S (d) Mg, K. Ca 4. Rank the ions in each set in order of decreasing size, and explain your ranking: (a) Se2, s, 02 (b) Te2, Cs", I
Step by Step Solution
3.39 Rating (158 Votes )
There are 3 Steps involved in it
3 A S Cl K B Mg Ca K 4 A Se S O Because we know as we move down the group size inc... View full answer

Get step-by-step solutions from verified subject matter experts


