Question: 1. Identify the oxidizing agent and the reducing agent in the reaction. 8H*(aq) + 6CI (aq) + Sn(s) + 4NO3 (aq) SnCl (aq) +
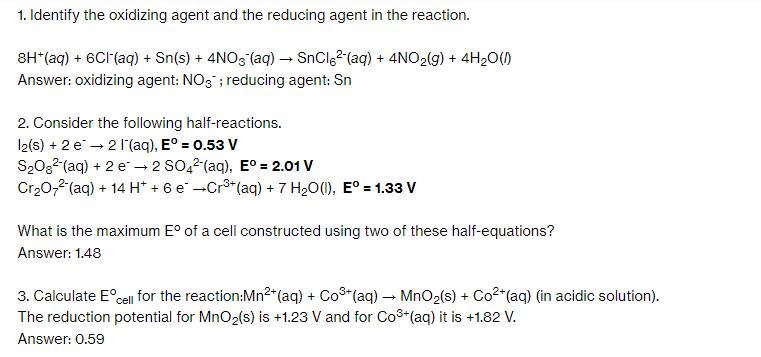
1. Identify the oxidizing agent and the reducing agent in the reaction. 8H*(aq) + 6CI (aq) + Sn(s) + 4NO3 (aq) SnCl (aq) + 4NO(g) + 4H0(1) Answer: oxidizing agent: NO3; reducing agent: Sn 2. Consider the following half-reactions. 12(s) + 2 e 2 l'(aq), E = 0.53 V SO (aq) + 2 e 2 SO42 (aq), E = 2.01 V CrO7 (aq) + 14 H+ + 6 e Cr+ (aq) + 7 HO(1), E = 1.33 V What is the maximum E of a cell constructed using two of these half-equations? Answer: 1.48 3. Calculate Ecell for the reaction:Mn+ (aq) + Co+ (aq) MnO(s) + Co+ (aq) (in acidic solution). The reduction potential for MnO(s) is +1.23 V and for Co+ (aq) it is +1.82 V. Answer: 0.59
Step by Step Solution
There are 3 Steps involved in it
In the given reaction 8Haq 6Claq Sns 4NO3aq SnCl2aq 4NO... View full answer

Get step-by-step solutions from verified subject matter experts


