Question: 1. Identify the run with the highest % error in the R value. What appears to be the major source of determinate error in this
1. Identify the run with the highest % error in the R value. What appears to be the major source of determinate error in this result? 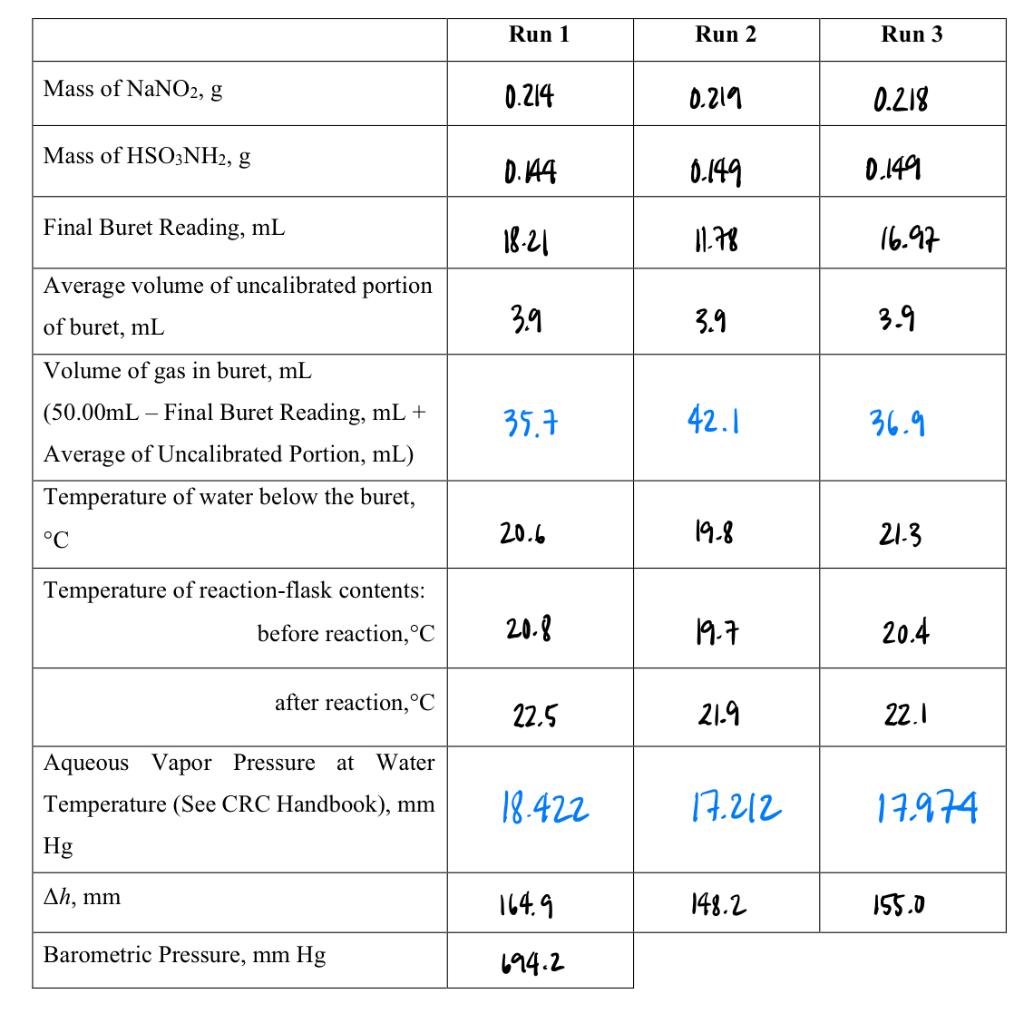
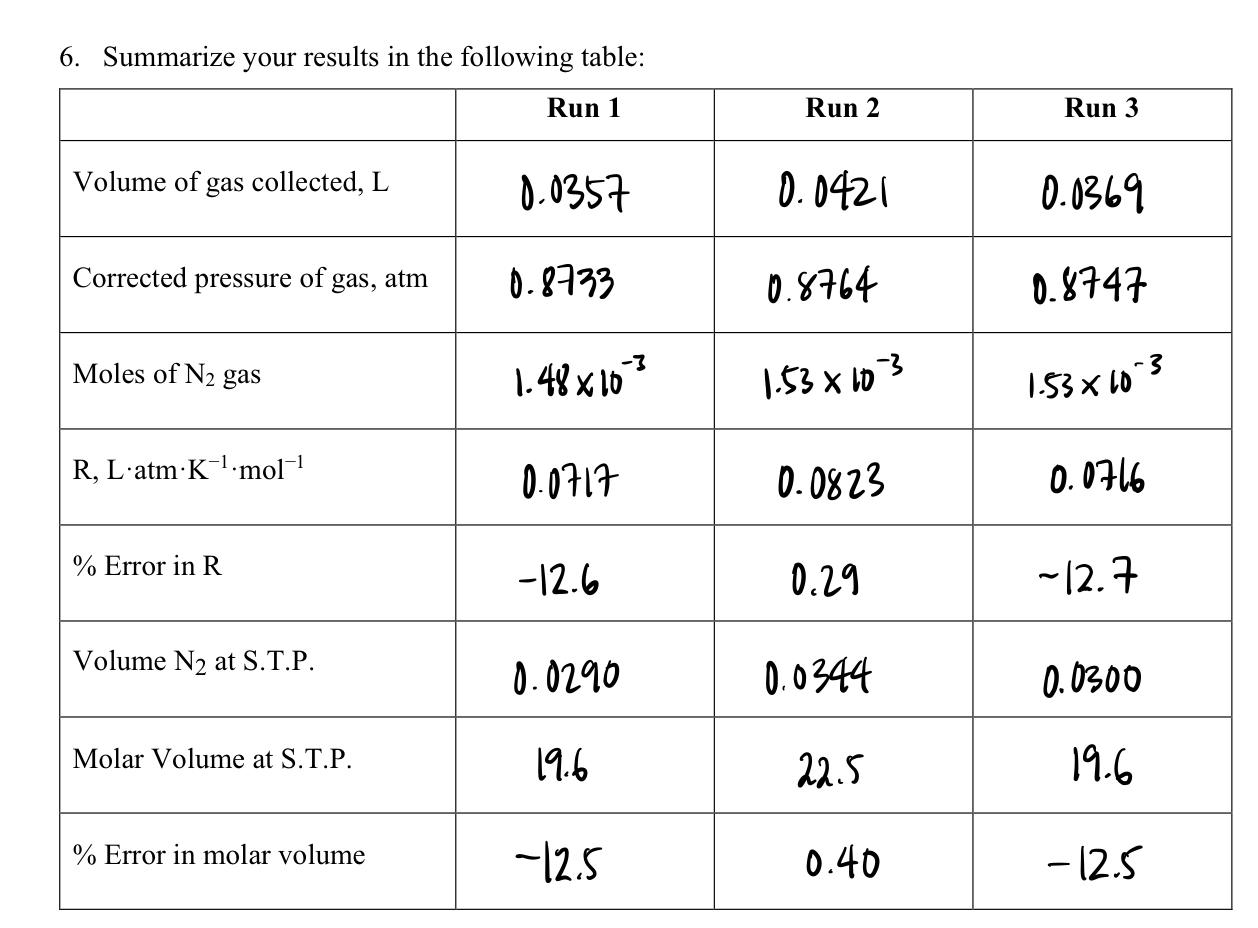
Run 1 Run 2 Run 3 Mass of NaNO2, g 0.214 0.219 0.218 Mass of HSO3NH2, g 0.144 0.149 0.149 Final Buret Reading, mL 18-21 11.78 16.97 3.9 3.9 3.9 Average volume of uncalibrated portion of buret, mL Volume of gas in buret, mL (50.00mL - Final Buret Reading, mL + Average of Uncalibrated Portion, mL) Temperature of water below the buret, 35.7 42.1 36.9 C 20.6 19-8 21-3 Temperature of reaction-flask contents: before reaction, C 20.8 19-7 20.4 after reaction, C 22.5 21.9 22.1 Aqueous Vapor Pressure at Water Temperature (See CRC Handbook), mm Hg 18.422 17.212 17.974 Ah, mm 164.9 148.2 155.0 Barometric Pressure, mm Hg 694.2 6. Summarize your results in the following table: Run 1 Run 2 Run 3 Volume of gas collected, L 0.0357 0.0421 0.0369 Corrected pressure of gas, atm 0.8733 0.8764 0.8747 Moles of N2 gas -3 1.48 x 10 1.53 x 103 1-53x103 R, L'atmK-.mol-! 0.0717 0.0823 0.0716 % Error in R -12.6 0.29 ~12.7 Volume N2 at S.T.P. 0.0290 0.0344 0.0300 Molar Volume at S.T.P. 19.6 22.5 19.6 % Error in molar volume -12.5 0.40 - 12.5
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


