Question: 1. Label the following compounds as ionic or molecular and name them appropriately. 2. Write the number of valence electrons for each atom, total number
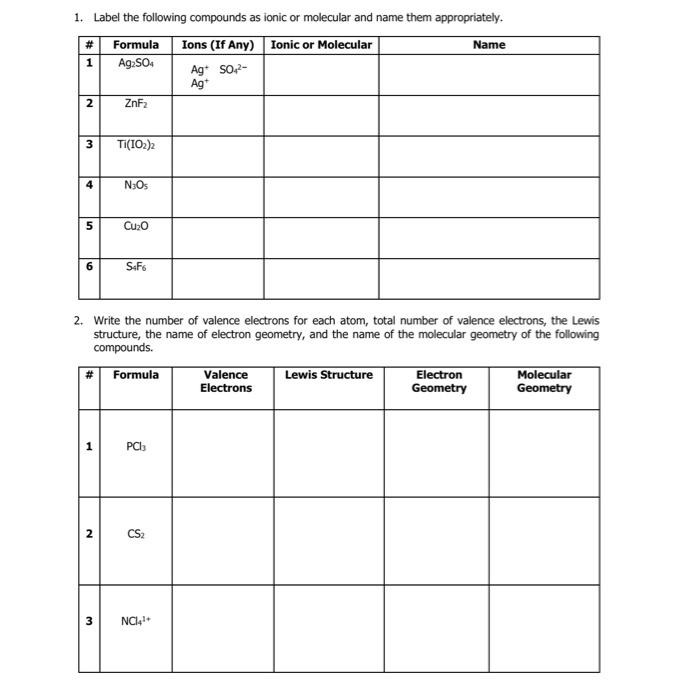

1. Label the following compounds as ionic or molecular and name them appropriately. 2. Write the number of valence electrons for each atom, total number of valence electrons, the Lewis structure, the name of electron geometry, and the name of the molecular geometry of the following compounds. 3. One of the goals of this lab is to become familiar with different shapes of simple molecules. a. What is the name of the theory used to predict electron and molecular geometries? b. Suppose a molecule consists of a central atom bonded to 3 outer atoms. There are no lone pairs on the central atom. What is the name of the electron shape of this molecule? What is the name of the molecular shape? - Electron Geometry: - Molecular Geometry: c. Suppose a molecule consists of a central atom bonded to 2 outer atoms. There is one lone pair on the central atom. What is the name of the electron shape of this molecule? What is the name of the molecular shape? - Electron Geometry: - Molecular Geometry: d. Suppose a molecule consists of a central atom bonded to 4 outer atoms. There are no lone pairs on the central atom. What is the name of the electron shape of this molecule? What is the name of the molecular shape? - Electron Geometry: - Molecular Geometry: e. Suppose a molecule consists of a central atom bonded to 3 outer atoms. There is one lone pair on the central atom. What is the name of the electron shape of this molecule? What is the name of the molecular shape? - Electron Geometry: - Molecular Geometry
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


