Question: 1) Lewis Structure: 2) Electron pair geometry: Molecular geometry: 3) Dipole moment? 4) Hydrogen bonds? 5) Intermolecular forces: 1) Lewis Structure: 2) Electron pair geometry:
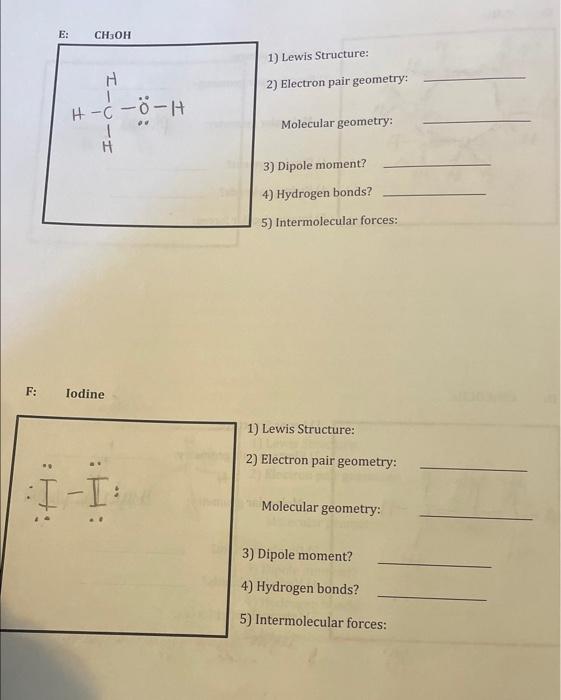

1) Lewis Structure: 2) Electron pair geometry: Molecular geometry: 3) Dipole moment? 4) Hydrogen bonds? 5) Intermolecular forces: 1) Lewis Structure: 2) Electron pair geometry: Molecular geometry: 3) Dipole moment? 4) Hydrogen bonds? 5) Intermolecular forces: 1) Draw out the Lewis structures. 2) Determine the electron pair geometry (name) and the molecular geometry (name and AXnEm classification of central atom(s) - one representative example is enough). 3) Determine whether the molecule possesses a dipole using molecular models. 4) Determine whether it is possible for the molecule to form hydrogen bonds. 5) List the different intermolecular forces present and identify the strongest one
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


