Question: 1 m 3 = 1 , 0 0 0 L Carnot cycle A Carnot cycle ( shown in the diagram on the right ) consists
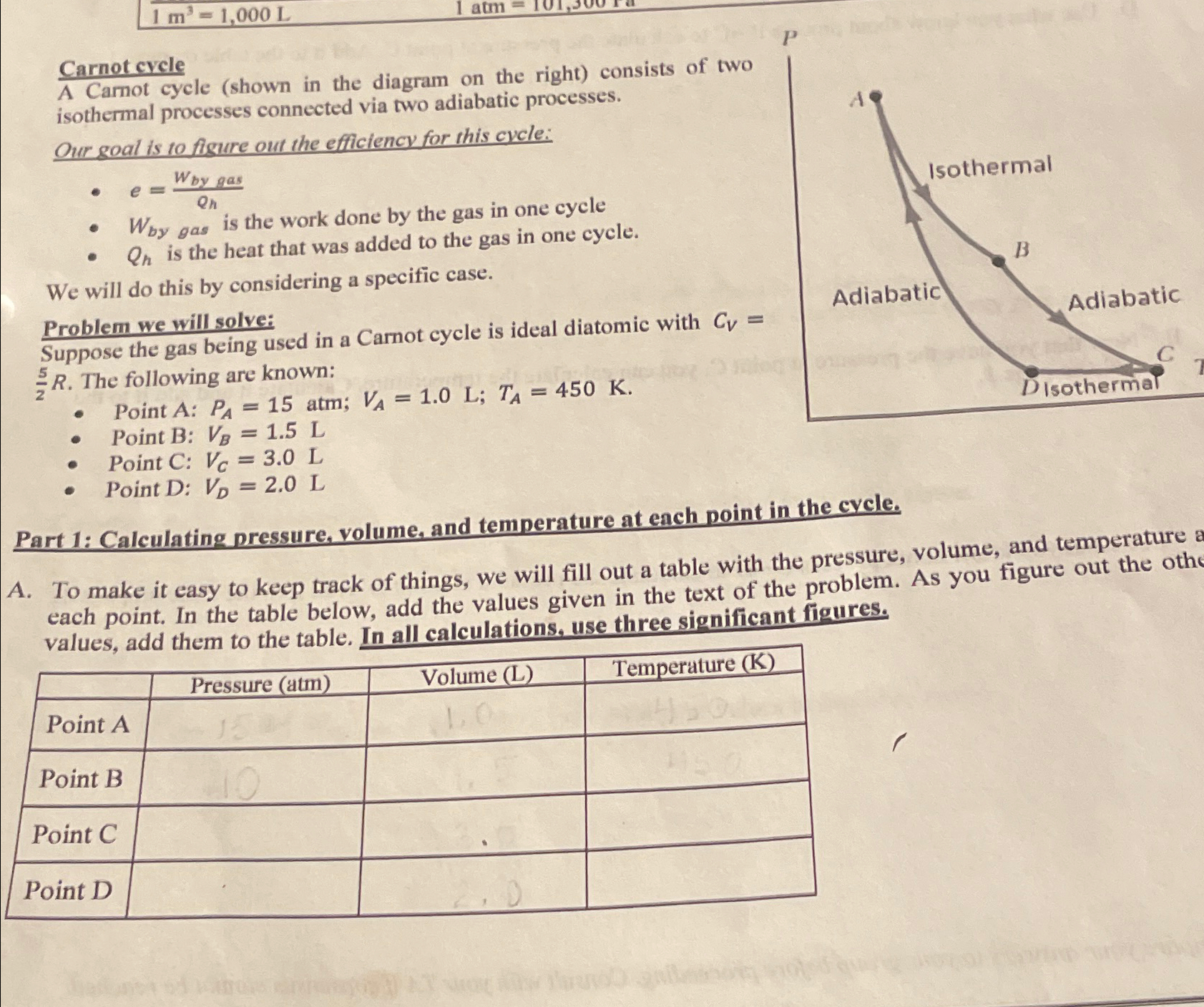
Carnot cycle
A Carnot cycle shown in the diagram on the right consists of two isothermal processes connected via two adiabatic processes.
Our goal is to fisure out the efficiency for this cycle:
is the work done by the gas in one cycle
is the heat that was added to the gas in one cycle.
We will do this by considering a specific case.
Problem we will solve:
Suppose the gas being used in a Carnot cycle is ideal diatomic with The following are known:
Point A: atm;;
Point B:
Point C:
Point D:
Part : Calculating pressure, volume, and temperature at each point in the cycle.
A To make it easy to keep track of things, we will fill out a table with the pressure, volume, and temperature each point. In the table below, add the values given in the text of the problem. As you figure out the oth values, add them to the table. In all calculations, use three significant figures.
tablePressure atmVolume LTemperature KPoint APoint BPoint CPoint D
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


