Question: 1 min 30 sec time, teme 530 95 L 95. 630 95 330 Don Demzed water Time 30 sec Hm 2.3 % 270 D 30.5
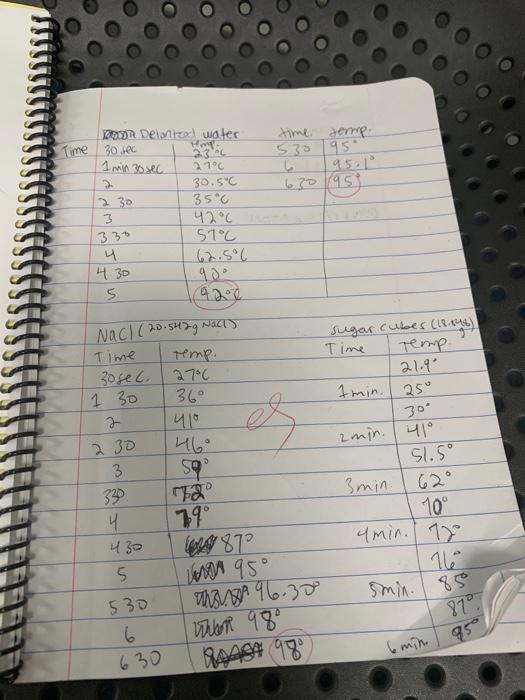


1 min 30 sec time, teme 530 95 L 95. 630 95 330 Don Demzed water Time 30 sec Hm 2.3 % 270 D 30.5" 30 35C 3 42C 51C 4 62.5C 430 5 424 Nac! (20.542g Nachy Time Temp. 30sec. 27C 1 30 7 410 2 30 46 3 592 360 es sugar cubes (18.146) Time Temp 21.90 1min. 25 30* zmin. | 41 51.50 3 min. 62 10 min. 18 16 D 430 5 19 CARA 872 W 95 USA 96.30 Wher 980 VAA04 98 smin. 185 530 6 630 8707 6 min. 95 Procedure: Measure out temperature Begin heatin intervals. In temperature time /temp 910 min. (9713 . Measure ou into a 250 for the Om Begin hear intervals. temperatu CARL CALCULATIONS Measure it into a 2 for the Begin be aterval mpera cul K P 64 Procedure: Measure out 125 ml of deionized water and add it into a 250 ml beaker. Record the initial temperature of the water and record it for the 0 min. reading in tedata table. Begin heating the solution on the hot plate. Record the time and temp. readings at 30 second intervals. In your notebook, record temp to the 0.1C. Continue until boiling until the temperature remains constant for at least 3 readings Measure out 125 ml of deionized water with approximately 20.g. of NaCl record mass) and add it into a 250 ml beaker. In your notebook, record the initial temperature of the water and record it for the 0 min. reading in the data table. Begin heating the solution on the hot plate. Record the time and temp. readings at 30 second intervals. In your notebook, record temp. to the 0.1C. Continue until boiling until the temperature remains constant for at least 3 readings. Measure out 125 mL of deionized water with approximately 6 sugar cubes (record mass) and add it into a 250 ml. beaker. In your notebook, record the initial temperature of the water and record it for the min. reading in tedata table. Begin heating the solution on the hot plate. Record the time and temp. readings at 30 second intervals. In your notebook, record temp to the 0.1C. Continue until boiling until the temperature remains constant for at least 3 readings. Calculations: To calculate actual motality, use the amount of sugar or NaCl added, convert to mols, m=mols solute/Kg solvent To calculate observed molality, use the formula AT. = i km: Use the observed boiling point and solve for m. Remember, 1 mol NaCl = 2 mol ions Construct a graph using the data collected. Plot temperature vs. time. Plot the data for all three samples. Use different colors or symbols to differentiate the three samples. 17
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


