Question: 1. (old exam problem). Consider the PT diagram for a pure substance to the right. (a) For each letter, indicate if it is a single
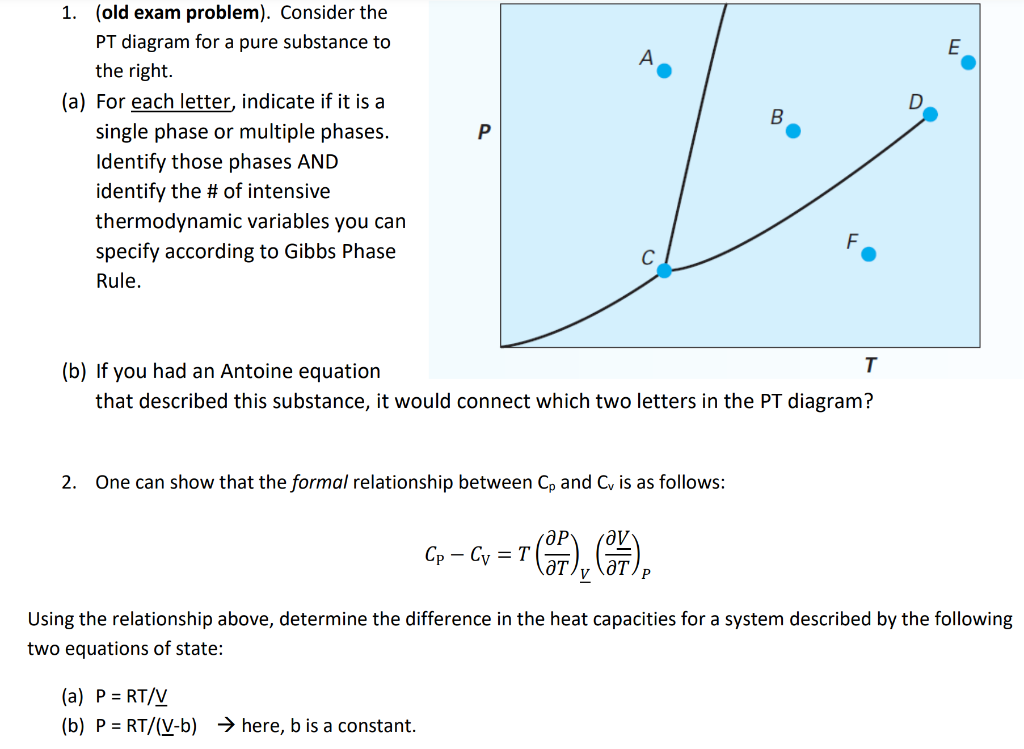
1. (old exam problem). Consider the PT diagram for a pure substance to the right. (a) For each letter, indicate if it is a single phase or multiple phases. Identify those phases AND identify the \# of intensive thermodynamic variables you can specify according to Gibbs Phase Rule. (b) If you had an Antoine equation that described this substance, it would connect which two letters in the PT diagram? 2. One can show that the formal relationship between Cp and Cv is as follows: CPCV=T(TP)V(TV)P Using the relationship above, determine the difference in the heat capacities for a system described by the following two equations of state: (a) P=RT/V (b) P=RT/(Vb) here, b is a constant
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


