Question: 1 Please try the problem again, your previous tries are listed below. A substance has the following properties: Heat capacities: 1.34J/gDC(golid)3.02J/gC(liquid)2.55J/gC(gas) Heat of Fusion =4.23kJ/
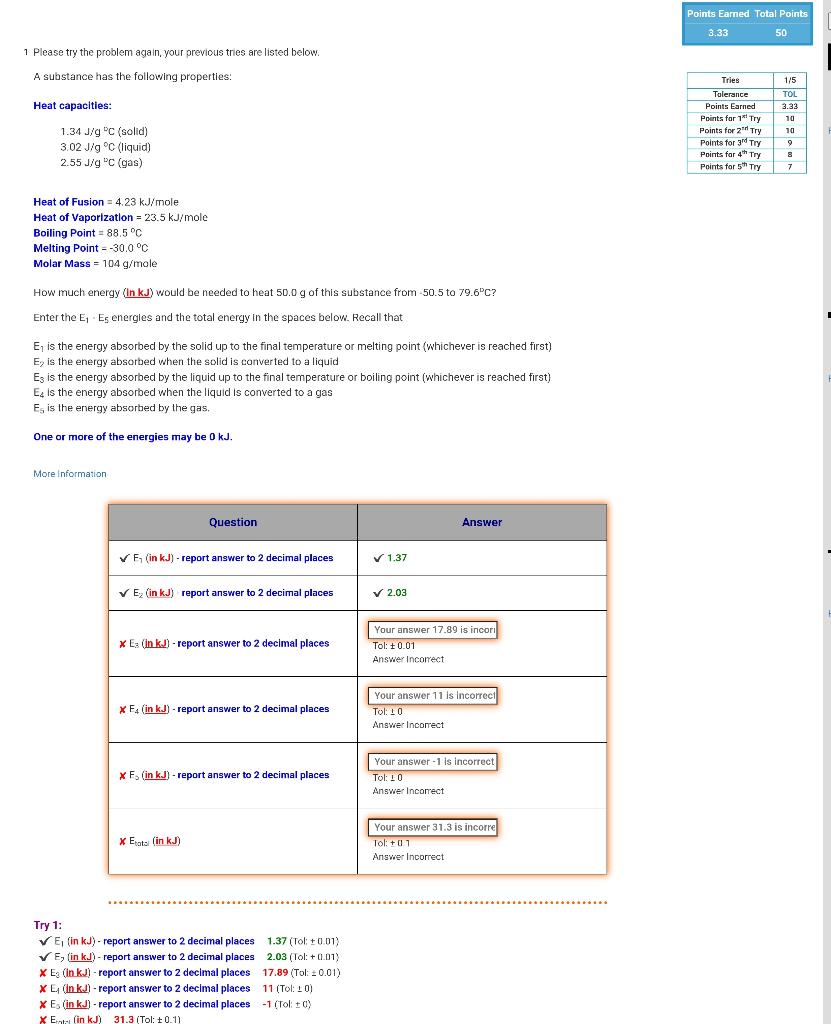
1 Please try the problem again, your previous tries are listed below. A substance has the following properties: Heat capacities: 1.34J/gDC(golid)3.02J/gC(liquid)2.55J/gC(gas) Heat of Fusion =4.23kJ/ mole Heat of Vaporizatlon =23.5kJ/ mole Boiling Point =88.5C Melting Point =30.0C Molar Mass =104g/ mole How much energy (lnkJ) would be needed to heat 50.0g of this substance from 50.5 to 79.6C ? Enter the E1E5 energles and the total energy in the spaces below. Recall that E1 is the energy absorbed by the solid up to the final temperature or melting point (whichever is reached first) E2 is the energy absorbed when the solid is conver ted to a liquid E3 is the energy absorbed by the liquid up to the final temperature or boiling point (whichever is reached first) E4 is the energy absorbed when the liquid is converted to a gas E5 is the energy absorbed by the gas. One or more of the energies may be 0kJ
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


