Question: 1- Reaction Rate Data Volume (mL) Mixture Average Time (min) Temp. (C) Acetone 4.0 M HCl 1.0 M 12 0.005 M H2O 1 10 10
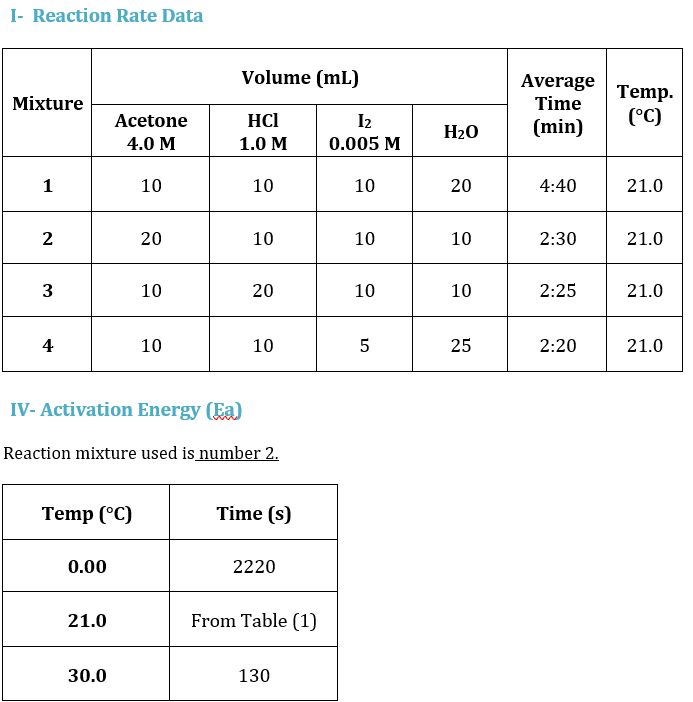
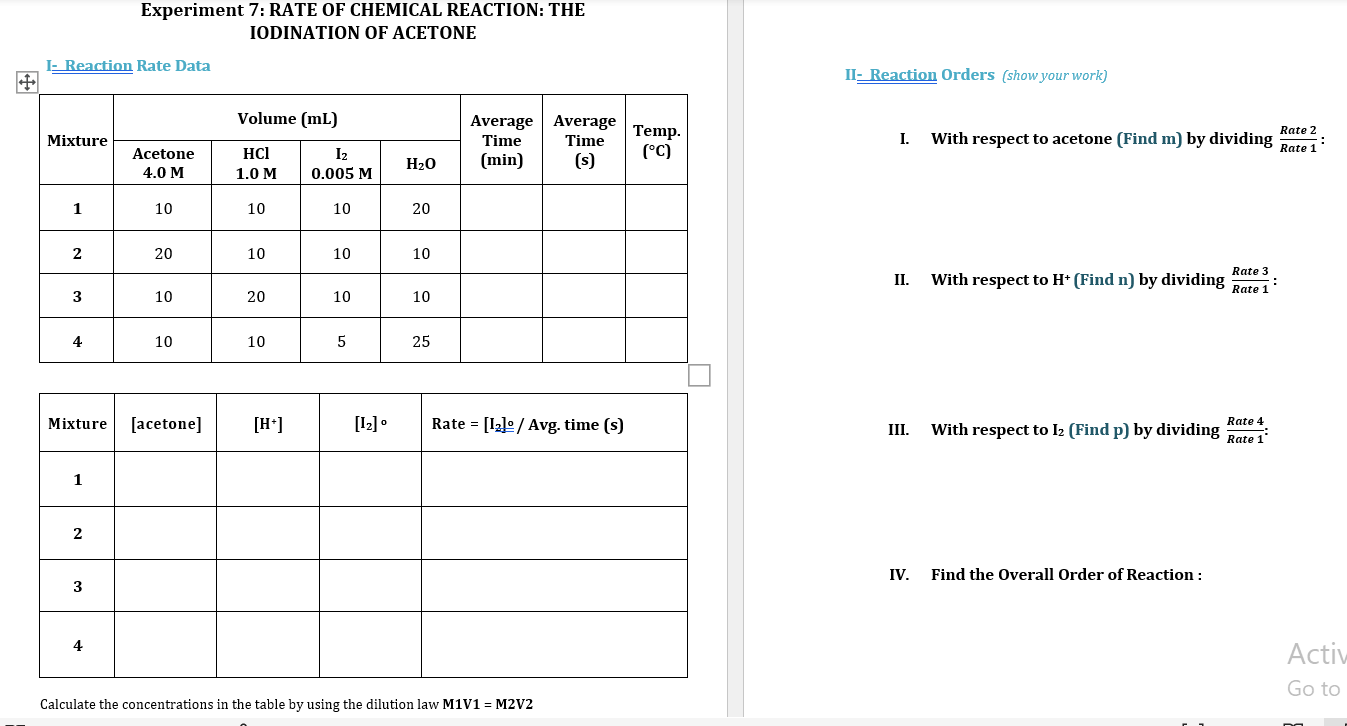
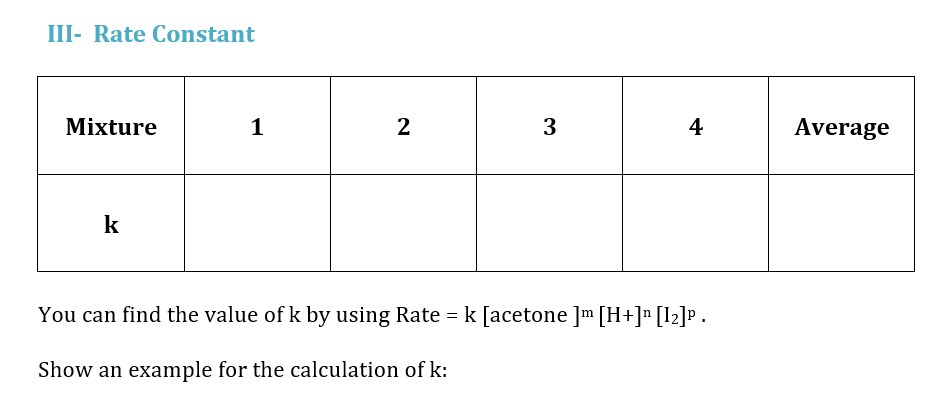
1- Reaction Rate Data Volume (mL) Mixture Average Time (min) Temp. (C) Acetone 4.0 M HCl 1.0 M 12 0.005 M H2O 1 10 10 10 20 4:40 21.0 2 20 10 10 10 2:30 21.0 3 10 20 10 10 2:25 21.0 4 10 10 5 25 2:20 21.0 IV- Activation Energy (Ea) Reaction mixture used is number 2. Temp (C) Time (s) 0.00 2220 21.0 From Table (1) 30.0 130 Experiment 7: RATE OF CHEMICAL REACTION: THE IODINATION OF ACETONE - Reaction Rate Data II- Reaction Orders (show your work) Volume (mL) Rate 2 Mixture Average Average Time Time (min) (s) Temp. (C) I. With respect to acetone (Find m) by dividing Rate 1 : Acetone 4.0 M HCl 1.0 M I2 0.005 M H2O 1 10 10 10 20 2 20 10 10 10 Rate 3 II. With respect to H+ (Find n) by dividing : 3 10 Rate 1 20 10 10 4 4 10 10 5 5 25 Mixture [acetone] [H] [12] Rate = [12] / Avg. time (s) Rate 4 III. ) With respect to 12 (Find p) by dividing Rate 1 1 1 2 IV. Find the Overall Order of Reaction : 3 4 Activ Go to Calculate the concentrations in the table by using the dilution law M1V1 = M2V2 III- Rate Constant Mixture 1 N 3 4 Average k You can find the value of k by using Rate = k [acetone ]m [H+]n [12]p. Show an example for the calculation of k
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


