Question: 1 [Review Topics) TUTOR Boiling Point Elevation (positive Kf) (References) A solution is prepared by dissolving 17.80 g of ordinary sugar (sucrose, C12H22011, 342.3 g/mol)
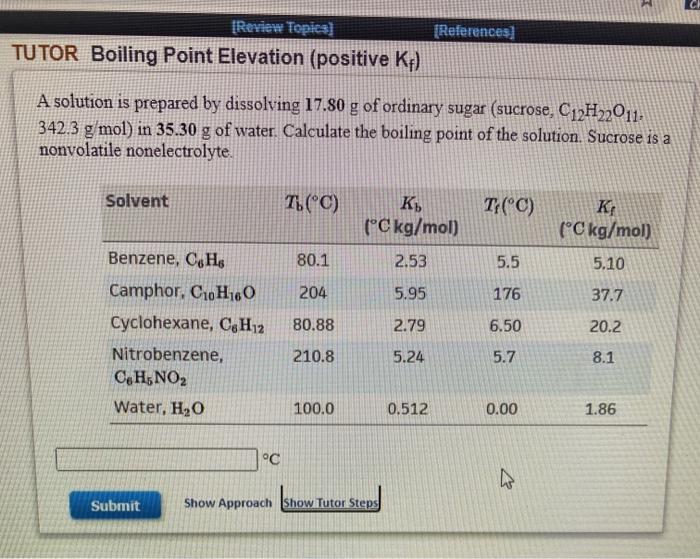
1 [Review Topics) TUTOR Boiling Point Elevation (positive Kf) (References) A solution is prepared by dissolving 17.80 g of ordinary sugar (sucrose, C12H22011, 342.3 g/mol) in 35.30 g of water. Calculate the boiling point of the solution. Sucrose is a nonvolatile nonelectrolyte Solvent T. (C) T(C) (C kg/mol) K: (C kg/mol) 80.1 2.53 5.5 5.10 204 5.95 176 37.7 Benzene, C6H Camphor, C10H160 Cyclohexane, CH12 Nitrobenzene, CH5NO2 Water, H20 80.88 2.79 6.50 20.2 210.8 5.24 5.7 8.1 100.0 0.512 0.00 1.86 C Submit Show Approach Show Tutor Steps
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


