Question: 1. Round Jet Diffusion Flame An ethaneitrogen mixture (50% ethane by volume) at 1 atm and room temperature exits a round tube of 5mm radius
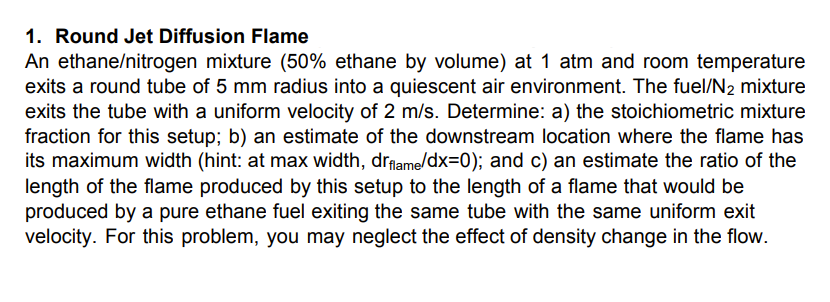
1. Round Jet Diffusion Flame An ethaneitrogen mixture (50\% ethane by volume) at 1 atm and room temperature exits a round tube of 5mm radius into a quiescent air environment. The fuel/ N2mixture exits the tube with a uniform velocity of 2m/s. Determine: a) the stoichiometric mixture fraction for this setup; b) an estimate of the downstream location where the flame has its maximum width (hint: at max width, drflame/dx=0 ); and c) an estimate the ratio of the length of the flame produced by this setup to the length of a flame that would be produced by a pure ethane fuel exiting the same tube with the same uniform exit velocity. For this problem, you may neglect the effect of density change in the flow
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


