Question: 1. Select the TRUE statements about the orbital below (y axis pointing to and away from you): A. Nodes (2 pt): a. It has 4
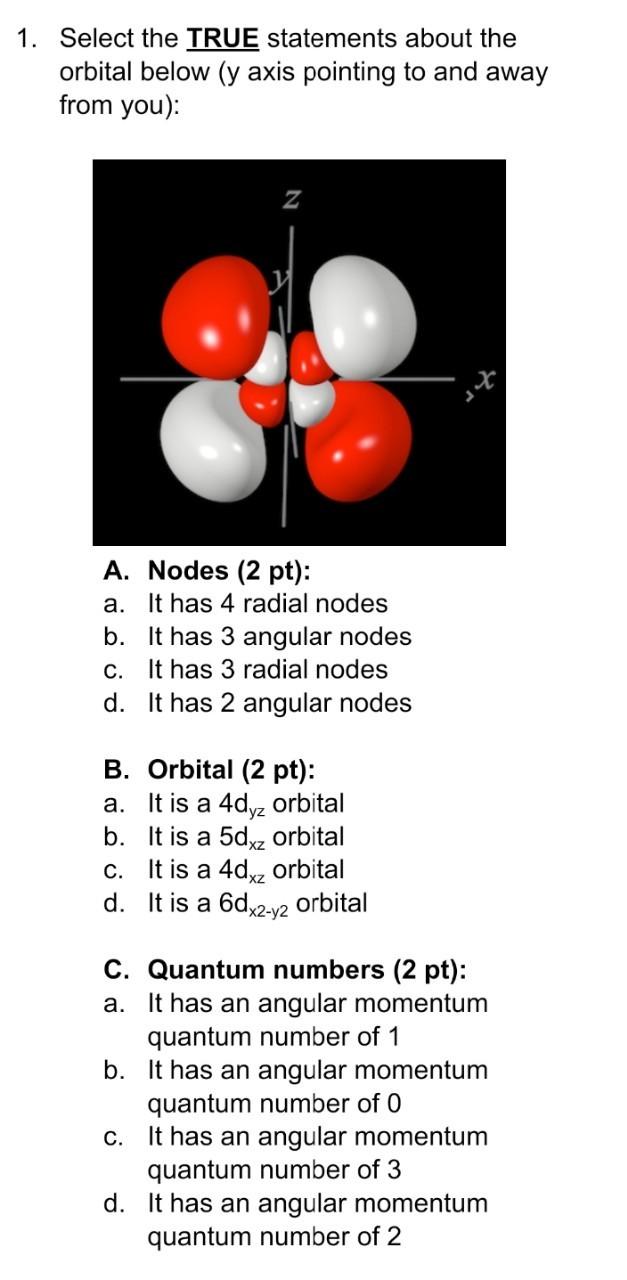
1. Select the TRUE statements about the orbital below (y axis pointing to and away from you): A. Nodes (2 pt): a. It has 4 radial nodes b. It has 3 angular nodes c. It has 3 radial nodes d. It has 2 angular nodes B. Orbital (2 pt): a. It is a 4dyz orbital b. It is a 5dxz orbital c. It is a 4dxz orbital d. It is a 6dx2-y2 orbital C. Quantum numbers (2 pt): a. It has an angular momentum quantum number of 1 b. It has an angular momentum quantum number of O c. It has an angular momentum quantum number of 3 d. It has an angular momentum quantum number of 2
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


