Question: 1. Select two compounds in Group 1, two compounds in Group 2, and two compounds in Group 3 to use in parts 1,2, and 3.
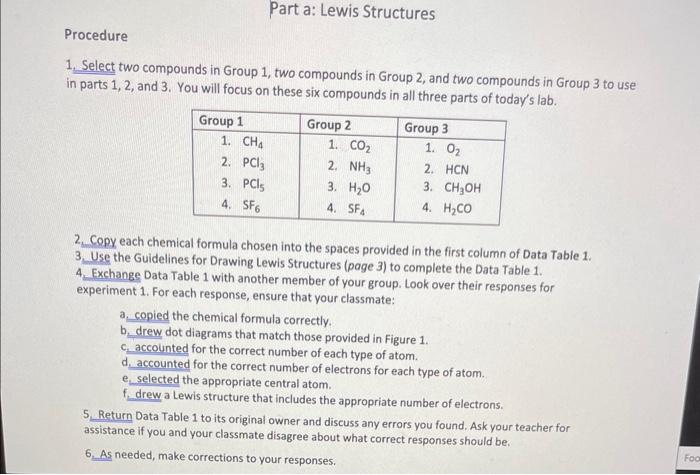
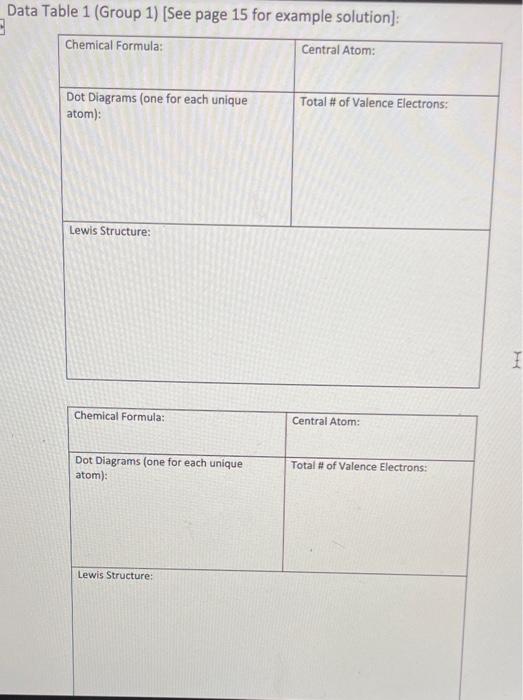
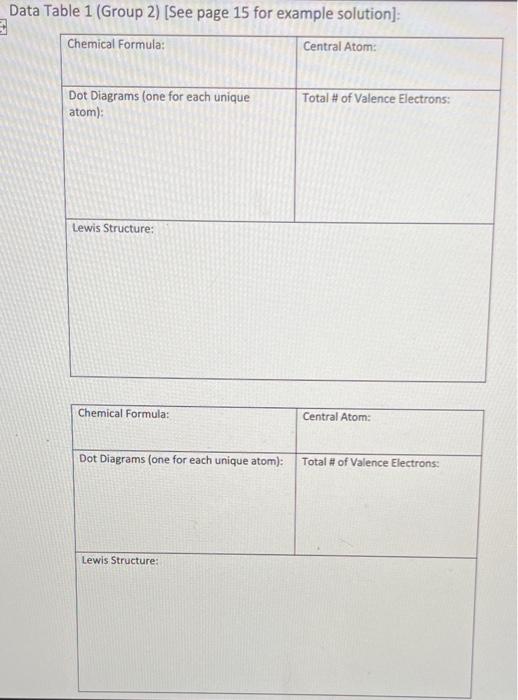

1. Select two compounds in Group 1, two compounds in Group 2, and two compounds in Group 3 to use in parts 1,2, and 3. You will focus on these six compounds in all three parts of today's lab. 2. Copy each chemical formula chosen into the spaces provided in the first column of Data Table 1. 3. Use the Guidelines for Drawing Lewis Structures (page 3) to complete the Data Table 1. 4. Exchange Data Table 1 with another member of your group. Look over their responses for experiment 1. For each response, ensure that your classmate: a. copied the chemical formula correctly. b. drew dot diagrams that match those provided in Figure 1. c. accointed for the correct number of each type of atom. d. accounted for the correct number of electrons for each type of atom. e selected the appropriate central atom. f drew a Lewis structure that includes the appropriate number of electrons. 5. Return Data Table 1 to its original owner and discuss any errors you found. Ask your teacher for assistance if you and your classmate disagree about what correct responses should be. 6. As needed, make corrections to your responses. Data Table 1 (Group 1) [See page 15 for example solution]: Data Table 1 (Group 2) [See page 15 for example solution]: Data Table 1 (Group 3) [See page 15 for example solution]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


